A 20. 0 g lead ball is heated in a Bunsen burner to 705 degrees celsius. It is then dropped into a 500. 0 g water bath. What is the initial temperature of the water if the final temperature is 35 degrees celsius? The C of lead is 0. 13 J/g degrees C.
[ Remember: Ch2o = 4. 18 J/g degrees celsius]
Answers
The initial temperature of the water is 25.8 °C. As a result, the lead ball loses heat rapidly when it is placed in the water bath, causing the water temperature to increase significantly.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical quantity that describes how hot or cold an object is. Temperature is usually measured using a thermometer and is commonly expressed in units such as degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K).
The energy gained by the water can also be calculated using the formula:
Q = mcΔT
where Q is the energy gained (in joules), m is the mass of the water (in grams), c is the specific heat capacity of water (in J/g°C), and ΔT is the change in temperature of the water (in °C).
We can calculate Q as follows:
Q = (500.0 g)(4.184 J/g°C)(35°C - T)
where T is the initial temperature of the water.
Since the energy lost by the lead ball is equal to the energy gained by the water, we can set these two equations equal to each other and solve for T:
(20.0 g)(0.13 J/g°C)(705°C - T) = (500.0 g)(4.184 J/g°C)(35°C - T)
Simplifying and solving for T gives:
T = 25.8°C
Therefore, the initial temperature of the water is 25.8 °C.
To know more about Temperature, visit;
https://brainly.com/question/26866637
#SPJ4
Related Questions
which are true about lubricants for cutting? they are composed almost entirely of hydrocarbons with some additives. they should be used at lower cutting speeds than other types of cutting fluids. they make cutting surfaces smoother. they dissolve easily in water they make it easier for cutting chips to not to stick to the cutting tool. they reduce the temperature of the cutting tool, but not as much as other cutting fluids.
Answers
A lubricant must keep its properties in the presence of additives and other items. Lubricating oil colours are more of a test for a grade or brand of oil's consistency than they are for its attributes. Thus, option B is correct.
What are the characteristics of lubricants?There are four different kinds of liquid lubricants. Mineral oil a. It is a material made from petroleum. These oils are stable at high temperatures because they have a high paraffine and naphthanic content.
Examples include steam cylinder oil, wire rope oil, refrigeration grade oil, gear oil, machine or engine oil, and circulating oil.
Therefore, they should be used at lower cutting speeds than other types of cutting fluids. They make cutting surfaces smoother.
Learn more about lubricants here:
https://brainly.com/question/30082267
#SPJ4
A compound has a emperical formula CH2 its Mr is28 find its molecular formula
Answers
Answer:
The molecular formula of the compound with an empirical formula of CH2 and a molar mass of 28 would be C2H4.
The instantaneous evaporation of liquid refrigerant in an evaporator is called _____.
Answers
The instantaneous evaporation of liquid refrigerant in an evaporator is called flash gas
What is a flash gas?
In refrigeration, flash-gas is refrigerant in gas form produced spontaneously when the condensed liquid is subjected to boiling.The presence of flash-gas in the liquid lines reduces the efficiency of the refrigeration cycle.It can also lead several expansion systems to work improperly, and increase superheating at the evaporator. This is normally perceived as an unwanted condition caused by dissociation between the volume of the system, and the pressures and temperatures that allow the refrigerant to be liquid. Flash-gas must not be confused with lack of condensation, but special gear such as receivers, internal heat exchangers, insulation, and refrigeration cycle optimizers may improve condensation and avoid gas in the liquid lines.To know more about liquid refrigerant, refer:
https://brainly.com/question/16493512
#SPJ4
DESPERATE WILL GIVE BRAINLIST AND THANKS (PLEASE HURRY )
Look at the Punnett square below which shows the cross between two frogs. Green is the dominant skin color while brown is the recessive skin color. What are the phenotype probabilities?
Question 8 options:
50% green, 50% brown
25% green, 25% brown
100% green, 0% brown
75% green, 25% brown

Answers
B.Green is more common than brown so more green genes are passed on. (25%)
C.The gene for green color is dominant over the gene for brown color. (0%)
D.The gene for brown color is dominant over the gene for green color. (100%)
I need help getting this done
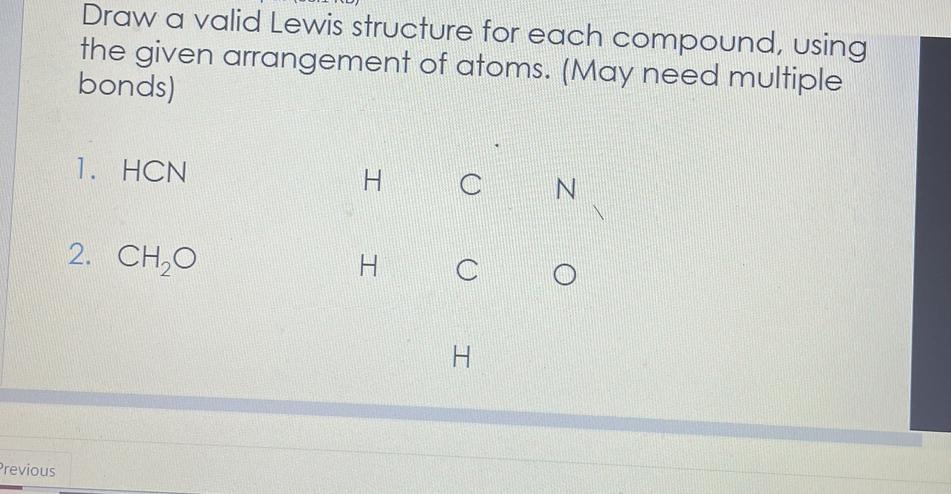
Answers
Answer:
I put the right structures in the pictures for you


Explain how you can tell air is a solution and not a colloid or suspension.
Answers
Answer:
air is a solution because it is homogeneous, uniform throughout,and doesn't scatter light
Explain why helium (He) is stable (unreactive), but hydrogen (H) is unstable (reactive)
Answers
Answer:
Helium is stable because it has 1 shell and the first shell can only have 2 electrons. Hydrogen is unstable because it has 1 shell but only 1 electron.
After the HCl and NaOH react, Fernando measures the
mass again. Using the mass before the reaction in the
diagram, what is the mass after the reaction?
Remember, It is in a closed system.
A. 5.00 grams
OB. 10.00 grams
O C. 15.00 grams
OD. 20.00 grams
Answers
Answer:c
Explanation:
As the combined mass of the HCl and NaOH is 15 grams before the reaction. Therefore the mass after the reaction will be 15 grams according to the law of conservation of mass. Therefore, option (C) is correct.
What is law of conservation of matter?Matter can be transformed form via physical changes and chemical changes from one form to another form, during any of these changes, the total mass is conserved. The same quantity of matter exists before and after the chemical or physical as none of the matter is created or destroyed.
The balanced equation between the reaction of HCl and NaOH:
\(HCl +NaOH \longrightarrow H_2O +NaCl\)
According to the law of conservation of mass, the mass of HCl and NaOH will be equal to the mass of the products water and NaCl.
As mentioned in the question the combined mass of HCl and NaOH measured before the reaction is 15 grams. Therefore, the mass of the products in the closed container will be equal to 15 grams as well.
Learn more about law conservation of matter, here:
brainly.com/question/23910777
#SPJ5
Your question was incomplete, most probably the complete question was,
Fernando places 15 ml of HCl and 50 ml of NaOH in 100 ml of a beaker. He places them on a scale together and measures the combined mass of 15 grams.
After the HCl and NaOH react, Fernando measures the mass again. Using the mass before the reaction, what is the mass after the reaction? Remember, It is in a closed system.
A. 5.00 grams
B. 10.00 grams
C. 15.00 grams
D. 20.00 grams
what does anhydrous mean in your own words?
Answers
Answer: no water
Explanation:
Answer:
a substance that is a crystalline compound, contains no water
PLSSS ANSWERRRRR????

Answers
Which of the following molecules or ions has a bent shape?
A: CIO4
B: PH3
C: OF2
D: PCI5
Answers
CAN SOMEONE EXPLAIN WHY IS THE ANSWER CARBONIC ACID AND NOT PHOSPHORIC ACID?
Which of the following acids does not form acidic salts?
a-Phosphoric acid
b-Carbonic acid
c-Hydrochloric acid
d-Sulphuric acid
Answers
Answer
Because carbonic acid is a weak acid.
A weak acid is only partially ionized in a solution.in other words,a weak acid doesn't completely dissociate or break apart into ions in a solution.
Mark the rule
Stronger acids form acidiy salts .Weaker acids from basic salts .Carbonic acids contain C means it is too weak .
So it can't form acidic salts
when magnesium loses electrons to become an ion, what does it become?
Answers
Answer:
It becomes an a positive ion (cation)
Explanation:
In order to become an ion, atoms should lose or gain electrons.
magnesium is losing 2 electrons which means that the ratio of protons is more than electrons. (proton-positive , electron-negative)
Magnesium has 12 electrons (electronic configuration: 2,8,2) which means it needs to lose 2 electrons. After it loses 2 electrons, there will be 12 protons and 10 electrons and since there is more protons it becomes positively charged.
Making it Mg²⁺
When magnesium loses two electrons, it becomes a magnesium ion with a 2+ charge, often denoted as Mg2+. The magnesium ion has a stable electron configuration and is attracted to negatively charged ions or molecules, such as chloride (Cl-) or oxygen (O2-).
Magnesium (Mg) is a metallic element that belongs to the second group of the periodic table. It is a highly reactive metal and easily loses two electrons to form an ion with a positive charge.
Magnesium ions are essential for many biological processes, including muscle contraction, nerve function, and the synthesis of DNA and RNA. They are also used in various industrial applications, such as the production of aluminum alloys and the purification of molten iron.
Know more about "electrons":-
https://brainly.com/question/30620848#
#SPJ11
Which one is the catalyst?
a.) the three dot one
b.) the two gray dots
c.) the one white dot
d.) the white and grey dot one

Answers
Answer:
the catalyst is the two gray dots
what's the advantage and disadvantage of using a stopwatch, clamp, thermometer, butanol, methanol, ethanol, propanol and other equipment from the lab
please help me
Answers
What is the formula to determine the mass in grams of 3.6 mols of H2SO4
Answers
The molecular mass of 3.6 moles of H₂SO₄.
So let us first calculate the molecular mass of 1 mole of H₂SO₄ and then when we calculate the molecular mass of 1 mole of H₂SO₄, we will calculate the molecular mass of 3.6 moles of H₂SO₄ by multiplying the molecular mass of 1 mole of H₂SO₄ by 3.6
Molecular mass of 1 mole of H₂SO₄ = 2*(molecular mass of Hydrogen) + (molecular mass of Sulphur ) + 4*(molecular mass of oxygen )
Molecular mass of 1 mole of H₂SO₄ = 2*1 + 32 + 4*16 = 98 grams
Mass of 3.6 moles H₂SO₄ = 3.6*98 =352.8 grams
To learn more about the mole concept, please refer to the following link -https://brainly.com/question/9758790
Why are some isotopes radioactive and some are not?
Answers
Answer: there are atoms that either have too many or too few neutrons or protons in their nuclei. This results in an imbalance between the jedi forces holding them together, which leads to an excess of internal energy. Such atoms are said to be unstable or radioactive.
Explanation:
f
501
cm3 of
hydrogen
are
collected
at
25
oC
and
100.3
kPa,
how
many
cm3 will
the
gas
occupy
at
STP?
Answers
Gas volume at STP was 1.12 10 7 cm3. At STP, the volume for 1 mole per gas is calculated to be 25 litres, followed by the kind of gas and dominant force. Actual gas having a repulsive force of +ve deviation.
What does STP stand for?V=nRT/P is how this formula is written. V = n R T / P, where V is the gas's volume in L, n is indeed the number of moles, R is the real gases constant, T is indeed the gas' temperature in K, and P is the gas's pressure in atm.
Is STP 22.4?S.T.P. One mole of every gas takes up 22.4 litres of space at typical conditions of temperature and pressure of 0o and 1 atm, respectively. This volume is an approximate value, since the volume of various gases varies significantly. Every gas at S.T.P. has a molar volume of 22.4L.
To know more about gas visit:
https://brainly.com/question/14812509
#SPJ1
Ms. Terries was given a beautiful deep red gem. She knew she might be holding a ruby. Or, she might be holding a stone worth more than rubies, a stone rared than diamonds – a red beryl. Ms. Terries needed to be careful in her calculations. Red Beryl is a mixture of minerals and its density can be anywhere from 2.63g/cm3 to 2.92 g/cm3 Rubies can also vary in density from 3.9 g/cm3 to 4.1 g/cm3. Ms. Terries weighed the stone. It weighed 4.35 g. When put into a graduated cylinder of water, the water level rose 1.5 mL. Determine the density of the gem. Was it ruby or red beryl?
Answers
Answer:
red beryl
Explanation:
Red Beryls = Density range is 2.63g/cm3 to 2.92 g/cm3
Rubies = Density Range is 3.9 g/cm3 to 4.1 g/cm3
Mass of stone = 4.35 g
Volume of solid = change in water level = 1.5 mL = 1 cm3
Relationship between mass, volume and density is given by;
Density = Mass / Volume
Density = 4.35 / 1.5 = 2.9 g/cm3
The density of the stone falls in the red beryls range hence it is a red beryl.
A large rift valley can be found along the east coast of Africa. It has been slowly widening over time, and it is now wide enough to contain many large lakes.
Which of these best explains the slow widening of this rift valley over time?
Group of answer choices
Earth's rotation
wind and water erosion
the Moon's gravitational pull
lithospheric plate movement
Answers
Answer:
wind and water erosion
Answer:
C is the answer
In the following acid-base reaction,H3O+ is theHCl(g) + H2O(1)→H30+(aq) + CI-(aq)А.BСacidconjugateacidconjugatebase

Answers
When we have an acid-base reaction, the acid will be that substance that donates protons, in the form of H+ ions. The base will be the one that accepts the H+ ions. Now, the base that accepts the protons becomes a potential proton donor, thus becoming a conjugate acid. This is the case with H3O+.
H2O accepts H+ ions, so it is a base and when it becomes H3O+ it becomes a potential H+ proton donor, that is, a conjugate acid.
so, the answer will be B. Conjugate acid
The molecular mass of methyl ethanoate is 75. 1 amu. Calculate the molecular mass of propanoic acid, an isomer of methyl ethanoate.
Answers
Molecular mass of propanoic acid an isomer of methyl ethanoate. is 74.1 amu or 74.1 g/mol.
What does isomer mean?Isomers can be defined as molecules with the same number of atoms of the same element but with different structural arrangements and properties.
Since the molecular formulas are the same for isomers, they have the same mass. Methyl ethanoate is an isomer of propionic acid so it has the same mass.
there's two type of isomer, geometric isomers and structural isomers
Geometric isomers are molecules that have the same chemical formula but different atomic arrangements. Geometric isomers have different physical and chemical properties.
An interesting property of geometric isomers is that their groups cannot switch positions independently.
Structural isomers are substances with the same chemical formula but different atomic bonds. Examples of structural isomers are the already mentioned chemicals n-butane and isobutane.
Since the molecular formulas are the same for isomers, they have the same mass.
Methyl ethanoate is an isomer of propionic acid so it has the same mass.
learn more about isomer at https://brainly.com/question/28188244
#SPJ4

Reaction 1: Add Zinc to Copper Sulfate (continued)
React
Observe Record observations of the reaction
and products in the data table.
The brown solid that formed is
DONE
Intro
Answers
During this procedure, the blue (CuSO 4) solution turns into a colorless ZnSO 4 solutions. balanced response. Copper sulfate is produced when Zn (s) and CuSO 4 (aq) combine. Sulfated zinc.
What is involved in providing balanced responses?The number of variables on either side of a formula is equal in a balanced action. A chemical reaction's atoms, ions, all molecules are represented by a number called the stoichiometric ratio, that balances exact amounts of each element on the product and reactant sides of equation.
A balanced equation is exactly what?One such equation describes an element's total charge and number of atoms in both the products and reactants of a reaction. In other words, the mass and charge of both sides of a reaction are equal.
To know more about balanced reactions visit:
https://brainly.com/question/28990748
#SPJ4
Answer:
the answer is copper :)
Explanation:
the graph above describes the location of an electron in a hydrogen atom that is in the ground state. what conclusion can be drawn from the graph?
Answers
By analyzing the graph the conclusion that can be derived about the location of an electron in a hydrogen atom that is in the ground state is that the greatest probability of locating electron is at a distance of one Bohr radius from the nucleus.
Generally the Bohr radius is described as a physical constant that is used to represent the most probable distance between the electron and nucleus of a hydrogen atom at its ground state (which is the lowest energy level). The constant's value of Bohr radius is symbolized as a₀, and its value is approximately 5.29177210903(80) x 10⁻¹¹ meters (m).
Hence, the greatest probability of locating electron is at a distance of one Bohr radius from the nucleus.
The graph is given is the image attached below.
Learn more about Bohr radius from the link given below.
https://brainly.com/question/31131977
#SPJ1

If the atomic number of Lithium is 3 and its mass number is 7, how many protons, electrons, and neutrons does a neutral
atom of Li have
Answers
Answer:
Protons = 3
Neutrons = 4
Electrons = 3
Explanation:
For every atom, the atomic number is equal to the number of protons.
In a neutral atom (atom with no charge) the number of protons is equal to the number of electrons.
To calculate neutrons subtract the number of protons (atomic number) from the rounded molar mass:
7 - 3 = 4 neutrons
select all the statements that correctly represent the reagents and conditions required in the various steps in the synthesis of the target molecule from ethyl benzene.
1. HNO3, H2SO4; 2. NBS, light
1. HNO3, H2SO4; 2. Br2, FeBr3
1. Br2, FeBrg; 2. HNO3, H2SO4
1. HNO3, H2O;2. Br2, heat
Answers
In Step A, the reagent is NBS with benzoyl peroxide in CCl4 at 80°C.
In Step B, the reagent is NaOC2H5 in C2H5OH at 50°C.
Acne lesions can be effectively treated with benzoyl peroxide. It has no effect on antibiotic resistance. It can be used with salicylic acid, sulfur, erythromycin, clindamycin, or adapalene (antibiotics) (a synthetic retinoid). Benzoyl peroxide/clindamycin and adapalene/benzoyl peroxide are two frequent combination medicines, with adapalene being a chemically stable retinoid that may be coupled with benzoyl peroxide, unlike Tazarotene and tretinoin.
For the treatment of acne lesions, combination treatments such as benzoyl peroxide/clindamycin and benzoyl peroxide/salicylic acid tend to be somewhat more effective than benzoyl peroxide alone. In the United States, the combination tretinoin/benzoyl peroxide was authorized for medicinal use in 2021.
Learn more about benzoyl peroxide
https://brainly.com/question/14711646
#SPJ4
Citric acid (C6H8O7) is an important intermediate in the Krebs cycle and a triprotic acid. What is the normality of a solution made by weighing 128 g of citric acid into a volumetric flask and diluting it to 2 L with water
Answers
Answer:
2.67 N
Explanation:
The normality equation looks like this:
Normality = Molarity (M) x Number of Equivalents
In this formula, the number of equivalents represents how many moles of the acidic species exist in the molecule. In other words, how many hydrogen atoms are in citric acid? This value would be 8 equivalents (as denoted by the subscript).
So, to find the normality, you need to (1) convert grams C₆H₈O₇ to moles (via molar mass), then (2) calculate the molarity (via molarity equation using moles and volume), and then (3) calculate normality (via normality equation using molarity and # of eq.).
(Step 1)
Molar Mass (C₆H₈O₇): 6(12.011 g/mol) + 8(1.008 g/mol) + 7 (15.998 g/mol)
Molar Mass (C₆H₈O₇): 192.116 g/mol
128 g C₆H₈O₇ 1 mole
---------------------- x ---------------------- = 0.666 moles C₆H₈O₇
192.116 g
(Step 2)
Molarity = moles / volume (L)
Molarity = 0.666 moles C₆H₈O₇ / 2 L H₂O
Molarity = 0.333 M
(Step 3)
C₆H₈O₇ -----> 8 hydrogen atoms
Normality = molarity x number of equivalents
Normality = 0.333 M x 8
Normality = 2.67 N
What are the end products of anaerobic respiration in yeast cell ?
Answers
Answer:
glucose ethanol and co2
please follow me and mark it brainliest
Answer:
In anaerobic respiration yeast breaks down glucose, forming ethanol and carbon dioxide as its waste products. When the glucose is nearly used up, and provided that oxygen is present, yeast uses the ethanol as a respiratory substrate to produce carbon dioxide and water in aerobic respiration.
Explanation:
Hope it is helpful....
The novice nurse administers RBCs to a client. Which actions by the novice nurse are deemed safe by the nurse preceptor? (Select all that apply.)
Priming the intravenous tubing with 0.9% sodium chloride.
Obtaining and documenting a full set of baseline vital signs.
NOT setting the infusion rate to deliver blood within 6 hours - it should be 4 hours.
Also require large gauge catheters 20-24 gauge.
Should stay with client for first 15 minutes
Answers
According to the nurse preceptor, the new nurse adheres to a number of safe practices while administering red blood cells (RBCs) to a patient.
Based on the given options, the actions that are deemed safe by the nurse preceptor are:
Priming the intravenous tubing with 0.9% sodium chloride.Obtaining and documenting a full set of baseline vital signs.Setting the infusion rate to deliver blood within 4 hours instead of 6 hours.Using large gauge catheters (20-24 gauge). When giving red blood cells (RBCs) to a patient, the novice nurse follows a number of safe procedures, according to the nurse preceptor. To ensure appropriate flushing and lower the chance of an air embolism, the inexperienced nurse correctly primes the intravenous tube with 0.9% sodium chloride in the first step. The second step is for the inexperienced nurse to collect and record a complete set of baseline vital signs. This creates a baseline for monitoring the client's status both before and after the transfusion. Third, in accordance with the advised duration for safe administration, the nurse modifies the infusion rate to administer the RBCs in 4 hours as opposed to 6 hours. Fourth, the inexperienced nurse employs big gauge catheters (20-24 gauge) to promote quick and smooth blood product flow and reduce problems.
To learn more about RBC's, refer to:
https://brainly.com/question/19029068
#SPJ4
If a solution has a temperature of 255 k, what is its temperature in degrees celsius?
Answers
If a solution has a temperature of 255 k, then its temperature in degrees Celsius is - 18 °C
The temperature is defined as ,
Temperature is the degree of hotness or coldness of a body, measured in degrees Fahrenheit or degrees Celsius.
Calculation ,
The temperature is converted from degree Celsius to kelvin as ,
Let suppose temperature = X °C
Then temperature in kelvin = X °C + 273 = ( X + 273 ) K
If a solution has a temperature = 255 K
Temperature in degrees Celsius = 255 K - 273 = - 18 °C
To learn more about solution please click here ,
https://brainly.com/question/7932885
#SPJ4