A sample of helium gas occupies 12. 4 L at 23°C and 0. 956 atm. What volume will it occupy at 1. 20 atm assuming that the temperature stays constant?
Answers
Related Questions
Someone plz help me :(

Answers
Answer:
Descriptive investigations use careful observations and measurements to develop findings. Science journals, science logs, and field notebooks are some of the tools scientists use to gather information for descriptive investigations.
Explanation:
Give the set of four quantum numbers that could represent the last electron added (using the Aufbau principle) to the Cl atom. A) n = 3, 1-1,m1-1,m,-+ B)1-3, 1 = 0, m1-1,m,-- C)n=3, l = 2, m1 =1 , m,-+ D) n = 2, l= 1, ml 1, m,-
Answers
Therefore, option C is the correct set of quantum numbers for the last electron added to the Cl atom.
To determine the set of quantum numbers for the last electron added to a chlorine (Cl) atom, we need to follow the Aufbau principle, which states that electrons fill orbitals in order of increasing energy.
The electronic configuration of Cl is [Ne] 3s² 3p⁵, which means that the last electron added will occupy one of the 3p orbitals.
The set of quantum numbers that could represent the last electron added to the Cl atom is:
C) n=3, l=2, ml=1, ms=-1/2
Explanation:
1. n = 3 indicates that the electron is in the third energy level.
2. l = 2 indicates that the electron is in a p orbital (since l = 0, 1, or 2 for s, p, and d orbitals, respectively).
3.ml = 1 indicates that the electron is in one of the three p orbitals with magnetic quantum number of 1 (-1, 0, or 1).
4.ms = -1/2 indicates the electron has a spin of -1/2, as all electrons in p orbitals have opposite spin.
To know more about quantum refer here
https://brainly.com/question/54156636#
#SPJ11
if you wanted to produce 4 moles of so3 gas, how many moles of so2 would you need to react? (assume an unlimited supply of o2 gas.) italic 2 italic space s o subscript italic 2 italic space italic (g italic )italic space italic plus italic space o subscript italic 2 italic space italic (g italic )italic space italic rightwards arrow italic 2 italic space s o subscript italic 3 italic space italic (g italic )
Answers
If we wanted to produce 4 moles of SO3 gas, we needed to react: 4 moles of SO2. This is because SO2 to SO3 coefficient ratio is 2:2.
How many moles of SO2 would we need to react?To determine how many moles we need to react, first, we got to write the balanced chemical equation of this reaction:
2SO2(g) + O2(g) --> 2SO3(g)
The number of coefficients is equal to the ratio of moles. The ratio of SO2: SO3 is 2:2. As we want to produce 4 moles of SO3 gas, the amount of SO2 we would need to react will also be 4 moles. Hence, the correct answer is 4 moles of SO2.
Learn more about stoichiometry here https://brainly.com/question/16060223
#SPJ4
What volume of air is present in human lungs if 0.19 mol are present at 312K and 1.3 atm?
A. 0.066
B. 2.9L
C. 5.5L
D.3.7L
Answers
Answer:
The answer is D - 3.7 L.
Isotope A contains 56 protons and 80 neutrons. Isotope B contains 55 protons and 81 neutrons. Isotope C contains 57 protons and 80 neutrons. Isotope D contains 56 protons and 74 neutrons. Which are isotopes of the same element?
Answers
Answer:
isotope A and isotope D
Explanation:
why they contains the same number of protons
The alpha decay of a radioactive nuclide (X) emits a He-4 nucleus and produces an isotope of Superscript 235 subscript 92 upper U.. What is X?
Answers
Answer: Thus X is Plutonium
Explanation:
Alpha Decay: In this process, a heavier nuclei decays into lighter nuclei by releasing alpha particle. The mass number is reduced by 4 units and atomic number is reduced by 2 units.
General representation of alpha decay :
\(_{Z}^{A}\textrm{X}\rightarrow _{Z-2}^{A-4}\textrm{Y}+_2^4\textrm{He}\)
where Z = atomic number
A= mass number
X and Y = atomic symbol of elements
\(_{Z}^{A}\textrm{X}\rightarrow _{92}^{235}\textrm{U}+_2^4\textrm{He}\)
Thus \(_{94}^{239}\textrm{Pu}\rightarrow _{92}^{235}\textrm{U}+_2^4\textrm{He}\)
Thus X is Plutonium with atomic number 94 and mass number 239
Answer:
the answer is D on edge
Explanation:
If 26.4 g of hydrogen, H2, is produced from 100.0 g of methane, CH4, reacting with excess water as shown, what is the percentage yield? CH4(g) + H2O(g) → CO(g) + 3H2(g)
Answers
Answer: Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated, multiplied by 100%.
% yield
=
actual yield
theoretical yield
⋅
100
%
So, let's say you want to do an experiment in the lab. You want to measure how much water is produced when 12.0 g of glucose (
C
6
H
12
O
6
) is burned with enough oxygen.
C
6
H
12
O
6
+
6
O
2
→
6
C
O
2
+
6
H
2
O
Since you have a
1
:
6
mole ratio between glucose and water, you can determine how much water you would get by
12.0
g glucose
⋅
1 mole glucose
180.0 g
⋅
6 moles of water
1 mole glucose
⋅
18.0 g
1 mole water
=
7.20
g
This represents your theoretical yield. If the percent yield is 100%, the actual yield will be equal to the theoretical yield. However, after you do the experiment you discover that only 6.50 g of water were produced.
Since less than what was calculated was actually produced, it means that the reaction's percent yield must be smaller than 100%. This is confirmed by
% yield
=
6.50 g
7.20 g
⋅
100
%
=
90.3
%
You can backtrack from here and find out how much glucose reacted
65.0 g of water
⋅
1 mole
18.0 g
⋅
1 mole glucose
6 moles water
⋅
180.0 g
1 mole glucose
=
10.8
g
So not all the glucose reacted, which means that oxygen was not sufficient for the reaction - it acted as a limiting reagent.
Explanation:
predict the sign on the thermodynamic quantities listed below based on the scene representing a physical change in a piston-cylinder assembly.
Answers
Based on a scene showing a physical change in a piston-cylinder assembly, the thermodynamic numbers indicated below are 760 torr 760.
What elements make up the first rule of thermodynamics?The first law of thermodynamics states that the following equation describes how the change in internal energy relates to the heat exchanged by the system and the work performed on or by the system: U = Q + W, where Q represents the heat energy that the system exchanged.
What are the thermodynamic quantities' two components?Extensive and intensive thermodynamic quantities are traditionally separated into these two categories. While intensive quantities are independent of system size, extensive quantities grow linearly with system size.
To know more about thermodynamic visit:-
https://brainly.com/question/1368306
#SPJ4
what happens when two atoms form a chemical bond?
Answers
When two atoms form a chemical bond, they stick together to make a new substance. Think of it like holding hands. The atoms share electrons, which are like little magnets, to form the bond. This helps make the atoms more stable and happy. Now, they can join together to make a molecule, which is like a group of atoms that are friends. Just like a group of friends can do things together that they can't do alone, molecules can do things that individual atoms can't.
Chemical bonding is the process by which atoms combine to form new compounds. The type of bond formed depends on the electronegativity difference between the atoms involved. Ionic bonding involves the transfer of electrons, covalent bonding involves the sharing of electrons, and metallic bonding involves a sea of delocalized electrons.
The resulting compound is usually more stable than the individual atoms and has different physical and chemical properties. When two atoms form a chemical bond, they share or transfer electrons to achieve a more stable electron configuration.
Electrons are negatively charged particles that orbit around the nucleus of an atom. Atoms are most stable when their outermost electron shell, also called valence shell, is filled with the maximum number of electrons it can hold. For example, the first shell can hold up to two electrons, and the second shell can hold up to eight electrons.
Atoms can form chemical bonds in three ways: ionic bonding, covalent bonding, and metallic bonding.
In ionic bonding, one or more electrons are transferred from one atom to another, resulting in positively charged ions and negatively charged ions. The positively charged ion is called a cation, and the negatively charged ion is called an anion. The resulting compound is held together by the electrostatic attraction between the oppositely charged ions. For example, sodium (Na) and chlorine (Cl) atoms can form an ionic bond to form sodium chloride (NaCl), also known as table salt.
In covalent bonding, atoms share one or more electrons to achieve a more stable electron configuration. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms involved. In a polar covalent bond, the electrons are shared unequally between the atoms, resulting in a slight negative charge on one atom and a slight positive charge on the other. For example, water (H2O) is a polar covalent molecule because oxygen (O) is more electronegative than hydrogen (H), so the electrons are shared unequally. In a nonpolar covalent bond, the electrons are shared equally between the atoms. For example, methane (CH4) is a nonpolar covalent molecule because the electrons are shared equally between the carbon (C) and hydrogen (H) atoms.
In metallic bonding, positively charged metal ions are held together by a sea of delocalized electrons. The resulting metal is a lattice of positive ions surrounded by a cloud of negative electrons. This type of bonding gives metals their characteristic properties, such as malleability, ductility, and conductivity.
Here you can learn more about Chemical bonding
https://brainly.com/question/15444131#
#SPJ11
Which list only includes terms that describe oxygen, gas, and O2?
A. Compound, molecule, pure substance
B. Element, molecule, pure substance
C. Atom, compound, molecule
D. Atom, element, molecule
Answers
Oxygen is an element, gas is a molecule and O2 is a pure substance.
Hope this helps
Oxygen gas O2 is a compound, a molecule and a pure substance.
Oxygen gas is a compound because it contains more than one atom( two oxygen atoms) that are chemically combined together.
Oxygen gas is a molecule because it can exist independently as a unit and the oxygen atoms are covalently bound.
It is a pure substance because it contains only oxygen molecules.
Learn more: https://brainly.com/question/19922822
-. A 100.0 g Chunk of Aluminum with an Initial Temperature of 450.0 C° is added to 100.0
mL of Ethyl Alcohol with an Initial Temperature of 80.0 C° (A) Calculate Equilibrium
Temperature of the Mixture (B) Calculate the Heat Exchange of the System
BA) 198 C° B) 22770 J All answers Approx
D A) 110C° B) 10000 J All answers Approx
A) 150 C° B) 30000 J All answers Approx
A) 300 C° B) 15000 J All answers Approx
Answers
The negative sign means that heat was transferred from the aluminium piece to the ethyl alcohol. Consequently, the heat exchange of the system is roughly -22,770 J, or -2.28 x 10^4 J (to 2 significant figures).
What happens when 100g of 100 C boiling water is introduced to a calorimeter?The mixture's temperature rises to 20 C. The mixture in the calorimeter is then dipped into by a metallic block of mass 1 kilogramme at 10°C. The temperature rises to 19 C once thermal equilibrium has been reached.
Q = m * c * T, where Q is the heat exchanged, m is the mass of the substance, c is the specific heat capacity, and T is the change in temperature, is the formula used to determine the heat exchanged.
We can use the following formula to determine the equilibrium temperature:
m_al * c_al * (T_eq - T_al) = m_et * c_et * (T_et - T_eq)
By entering the specified values, we obtain:
(0.100 kg) * (0.902 J/g°C) * (T_eq - 450.0°C) = (0.100 kg) * (2.44 J/g°C) * (80.0°C - T_eq)
Simplifying, we get:
90.2 J/C * (T_eq - 450.0) = 244 J/C * (80.0 - T_eq)
90.2 T_eq - 40590 = 19520 - 244 T_eq
334.2 T_eq = 60110
T_eq ≈ 179.7°C ≈ 180°C (to the nearest 10°C)
As a result, the mixture's equilibrium temperature is roughly 180 °C.
(B) We can apply the same formula as before to determine the system's heat exchange. We can suppose that the mixture has a specific heat capacity equal to that of ethyl alcohol.
Q = m_al * c_al * (T_eq - T_al) + m_et * c_et * (T_eq - T_et)
Plugging in the given values, we get:
Q = (0.100 kg) * (0.902 J/g°C) * (180.0°C - 450.0°C) + (0.100 kg) * (2.44 J/g°C) * (180.0°C - 80.0°C)
Q ≈ -22,770 J ≈ -2.28 x 10⁴ J (to 2 significant figures)
To know more about ethyl alcohol visit:-
https://brainly.com/question/29153788
#SPJ1
how many atoms are in hydrogen
Answers
Answer:6.02
Explanation:
Answer:
there are about 6 atoms in hydrogen
which process moves ions from an area of high concentration?
Answers
The process that moves ions from an area of high concentration to an area of lower concentration is called diffusion.
Diffusion is the movement of particles or substances from an area of high concentration to an area of lower concentration until the concentration of the substance is uniform throughout the space.
Key points:
Diffusion occurs naturally as a result of the random thermal motion of particles.The rate of diffusion is proportional to the concentration gradient (difference in concentration between two areas) and the temperature.Diffusion occurs in liquids, gases, and through membranes.Diffusion plays a role in many biological processes, such as the movement of oxygen and carbon dioxide in the respiratory system, and the absorption of nutrients in the digestive system.Diffusion can also be used in various industrial and technological processes, such as the separation of components of a mixture, and the purification of substances.Learn more about Diffusion here:
https://brainly.com/question/8880115
#SPJ4
How do you identify conduction?
Answers
Direct contact between objects causes conduction or heat transfer. The heat is transferred inside the fluid during convection. Heat transfer in radiation happens by electromagnetic waves without the use of particles.
How can conduction be distinguished?First, ascertain whether the two things are in contact. If they are, conduction is how heat is transferred between them. Determine whether there is a fluid medium, such as a liquid or gas, connecting the items if they are not in contact.
How do you recognize conduction, a type of heat transfer?Evidence of heat transport is apparent. Convection is the phenomenon that causes the air to shimmer over radiators. Conduction is the phenomenon that causes you to feel warm when you place your hand on a spoon that has been sitting in a hot bowl of soup (radiation).
to know more about conduction here:
brainly.com/question/15085692
#SPJ4
Current Attempt in Progress Compound A has the molecular formula C7H12. Hydrogenation of compound A produces 2-methylhexane. Hydroboration oxidation of compound A produces an aldehyde. 09.47 Draw the structure of compound A
Answers
Structure of compound A: H2C=CH-CH=CH-CH2-CH2-CH3.
To draw the structure of compound A (C7H12), follow these steps:
1. Determine the degrees of unsaturation: The molecular formula of compound A is C7H12, which gives us 4 less hydrogens than the formula for an alkane with 7 carbons (C7H16). This indicates that compound A has two degrees of unsaturation.
2. Identify the functional groups: Since hydrogenation of compound A produces 2-methylhexane, this suggests that the molecule has an alkene. Additionally, hydroboration-oxidation of compound A produces an aldehyde, indicating that the alkene is terminal (at the end of the carbon chain).
3. Draw the basic structure: Start with a terminal alkene on a 7-carbon chain, giving us C6H11.
4. Add the remaining degree of unsaturation: Since there is still one more degree of unsaturation, we need to add a double bond or a ring. Since we know that hydrogenation produces 2-methylhexane, the double bond must be adjacent to the existing terminal alkene, forming a diene.
5. Finalize the structure: The structure of compound A is 1,3-heptadiene. It has a 7-carbon chain with a terminal alkene at the first carbon and another double bond at the third carbon.
Here's the structure of compound A: H2C=CH-CH=CH-CH2-CH2-CH3.
Learn more about degree of unsaturation.
brainly.com/question/30555776
#SPJ11
Use the portion of the periodic table shown at the bottom to answer the questions.
Part 1: Name two elements that have the same properties as magnesium (Mg).
Part 2: Determine the number of protons, electrons, and neutrons present in an atom of potassium (K). Explain how you determined your answer using complete sentences.

Answers
Answer:
Part 1 calcium and strontium have same properties as magnesium because all three are in same group.
Part 2 As atomic number of potassium is 19 so it contains 19 protons and 19 electrons because it is neutral
Potassium has 20 neutrons because its mass is 39
We can find neutron = atomic mass - atomic number
Explanation:
How would you change liquid water into ice? Be sure to explain what is happening to the energy (the heat)
Answers
Answer:
By putting the liquid water in the fridge
Explanation:
When it is solid the heat will melt to liquid water and if you put it inside a boiling kettle if it boil finished it will bring out gas,if you open it the cover of the kettle you see water if you put it in the fridge and it will be solid again.
the ions in calcium chloride dissociate in the presence of water. what is the cation that is produced in this reaction?
Answers
The cation that is produced when calcium chloride dissociates in the presence of water is Ca²⁺.
In the presence of water, calcium chloride dissociates into its constituent ions:
CaCl₂(s) + H₂O(l) → Ca²⁺(aq) + 2Cl⁻(aq)
As seen from the equation, the cation which is produced when calcium chloride dissociates in the presence of water is Ca²⁺.
Calcium chloride (CaCl₂) is a chemical compound that is commonly used in a variety of applications, such as in de-icing and dust control, as a food additive, and in water treatment.
It is a white crystalline solid that is highly soluble in water and has a salty taste. When calcium chloride is dissolved in water, it dissociates into calcium cations (Ca²⁺) and chloride anions (Cl⁻), making it an electrolyte. Calcium chloride is also used in the production of cement and as a desiccant to remove moisture from the air.
To know more about calcium chloride here
https://brainly.com/question/15296925
#SPJ4
1. For the balanced chemical equation below, write all possible mole ratios.
2Ca + O2 → 2CaO
2. Hydrogen and oxygen react under a specific set of conditions to produce water according to the following balanced equation:
2H2 + O2 → 2H₂O
How many moles of hydrogen would be required to produce 5.0 mol of water?
3. If 4.50 mol of ethane, C₂H6, undergoes combustion according to the balanced equation
below, how many moles of oxygen are required?
2C2H6+7024CO2 + 6H₂O
4. During lightning flashes, nitrogen combines with oxygen to form nitrogen monoxide, NO, which then reacts further with O2 to produce nitrogen dioxide, NO2.
a. Write the balanced equation for this reaction.
b. What mass of NO2 is formed when NO reacts with 384 g O₂?
c. How many grams of NO are required to react with this amount of O₂?
Answers
After considering all the given data we conclude that A) the ratios of the balanced chemical equation is 2:1, 2:2, 1:2, B) the number of moles of hydrogen needed is 0.5 mol H₂, C) moles of oxygen are required for the combustion is 15.8, the balanced chemical equation is N₂(g) + O₂(g) → 2NO(g), D) a)552 g NO₂ will be formed when NO reacts, b) NO reacts with 384 g O₂, c)360 g NO are required to react with 384 g O₂.
Here are the mole ratios for the balanced chemical equation below:
2Ca + O₂ → 2CaO
A) The mole ratio between Ca and O₂ is 2:1 since 2 moles of Ca were needed to produce 1 mole of O₂. The mole ratio between Ca and CaO is 2:2 since 2 moles of Ca were needed to produce 2 moles of CaO. The mole ratio between O₂ and CaO is 1:2 since 1 mole of O₂ was needed to produce 2 moles of CaO.
B)The balanced equation for the reaction between hydrogen and oxygen to produce water is:
2H2 + O₂ → 2H₂O
From this equation, we can see that 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water. Therefore, to produce 5.0 mol of water, we would need:
5.0 mol H₂O × (2 mol H₂ / 2 mol H₂O)
= 5.0 mol H₂
So we would need 5.0 moles of hydrogen to produce 5.0 moles of water.
C) The balanced equation for the combustion of ethane is:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
According to this equation, 7 molecules of oxygen are required for the complete combustion of 2 molecules of ethane1. Therefore, we can calculate how many moles of oxygen are required for the combustion of 4.50 mol of ethane as follows:
(7 mol O₂ / 2 mol C₂H₆) x (4.50 mol C₂H₆) = 15.8 mol O₂
Therefore, 15.8 moles of oxygen are required for the combustion of 4.50 mol of ethane.
D) a) The balanced equation for this reaction is:
N₂(g) + O₂(g) → 2NO(g)
b) To find out how much NO₂ is formed when NO reacts with 384 g O₂, we first need to find out how many moles of O₂ are present in that amount. We can use the molar mass of O₂ (32 g/mol) to convert grams to moles:
384 g O₂ × (1 mol / 32 g) = 12 mol O₂
From the balanced chemical equation above, we know that one mole of NO reacts with one mole of O₂ to form one mole of NO₂. Therefore, if we have 12 molO₂ present, then we will also form 12 mol NO₂.
To find out how much mass that corresponds to, we can use the molar mass of NO₂ (46 g/mol):
12 mol NO₂ × (46 g / mol) = 552 g NO₂
Therefore, 552 g NO₂ will be formed when NO reacts with 384 g O₂.
c) To find out how many grams of NO are required to react with this amount of O₂, we first need to find out how many moles of NO are required. From the balanced equation above, we know that one mole of NO reacts with one mole of O₂.
Since we have 12 mol O₂ present (as calculated in part b), then we will also need 12 mol NO.
To convert from moles to grams, we can use the molar mass of NO (30 g/mol):
12 mol NO × (30 g / mol) = 360 g NO
Therefore, 360 g NO are required to react with 384 g O₂.
To learn more about balanced chemical equation
https://brainly.com/question/26694427
#SPJ1
allows oxidation of co (carbon monoxide) to less-harmful co₂ (carbon dioxide) allows oxidation of hc (unburned hydrocarbons) to co₂ (carbon dioxide) and h₂o (water)
Answers
The oxidation of co (carbon monoxide) to less-harmful co₂ (carbon dioxide) and the oxidation of hc (unburned hydrocarbons) to co₂ (carbon dioxide) and h₂o (water).
What is meant by an oxidation reaction?Oxidation is that the loss of electrons during a reaction by a molecule, atom or ion. Oxidation occurs when the oxidation number of a molecule, atom or ion is increased. the other process is called reduction, which occurs when there's a gain of electrons or the oxidation state of an atom, molecule, or ion decreases.
Why is it called an oxidation reaction?
The term oxidation was first employed by Antoine Lavoisier to signify the reaction of a substance with oxygen. Much later, it had been realized that the substance, upon being oxidized, loses electrons, and therefore the meaning was extended to include other reactions in which electrons are lost, no matter whether oxygen was involved.
Learn more about oxidation reaction:
brainly.com/question/25886015
#SPJ4
A compound containing a functional group with a C-Z σ bond is often polar because the heteroatom Z is ______ electronegative than carbon. The atom Z has one or more lone pairs of electrons, allowing it to act as both a nucleophile and a _______
Answers
A functional group is a group of atoms within a molecule that is responsible for the characteristic chemical reactions of that molecule. When a functional group contains a C-Z σ bond, the compound is often polar because the heteroatom Z is more electronegative than carbon.
Electronegativity refers to the tendency of an atom to attract electrons towards itself when it is part of a chemical bond. Since the electronegativity of Z is higher than carbon, the electron density in the C-Z bond is shifted towards the Z atom, creating a partial positive charge on the carbon and a partial negative charge on the Z atom.
The atom Z in a functional group with a C-Z σ bond typically has one or more lone pairs of electrons. This makes it a nucleophile, meaning it is attracted to positively charged atoms or molecules and can donate its lone pair of electrons to form a new bond. The Z atom can also act as a leaving group, meaning it can dissociate from the molecule and take its lone pair of electrons with it.
Examples of functional groups with a C-Z σ bond include carbonyl groups (C=O), carboxylic acid groups (COOH), and amine groups (NH2). In each case, the Z atom is more electronegative than carbon, resulting in a polar molecule. The presence of lone pairs on the Z atom also allows it to participate in chemical reactions as both a nucleophile and a leaving group.
To know more about nucleophile
https://brainly.com/question/14052597
#SPJ11
Which of the following choices contains the most thermal energy?
a penny that is 20 degrees Celsius ( oC)
an atom of aluminum that is 20 degrees Celsius ( oC)
a 50 milliliter (ml) glass of water at 20 degrees Celsius ( oC)
a 900 milliliter (ml) pitcher of orange juice at 20 degrees Celsius ( oC)
Answers
Answer:
Honestly no idea
Explanation:
FOOOOOOOOD
Telescopes PLS missing words i've done most of it but stuck on two pls help
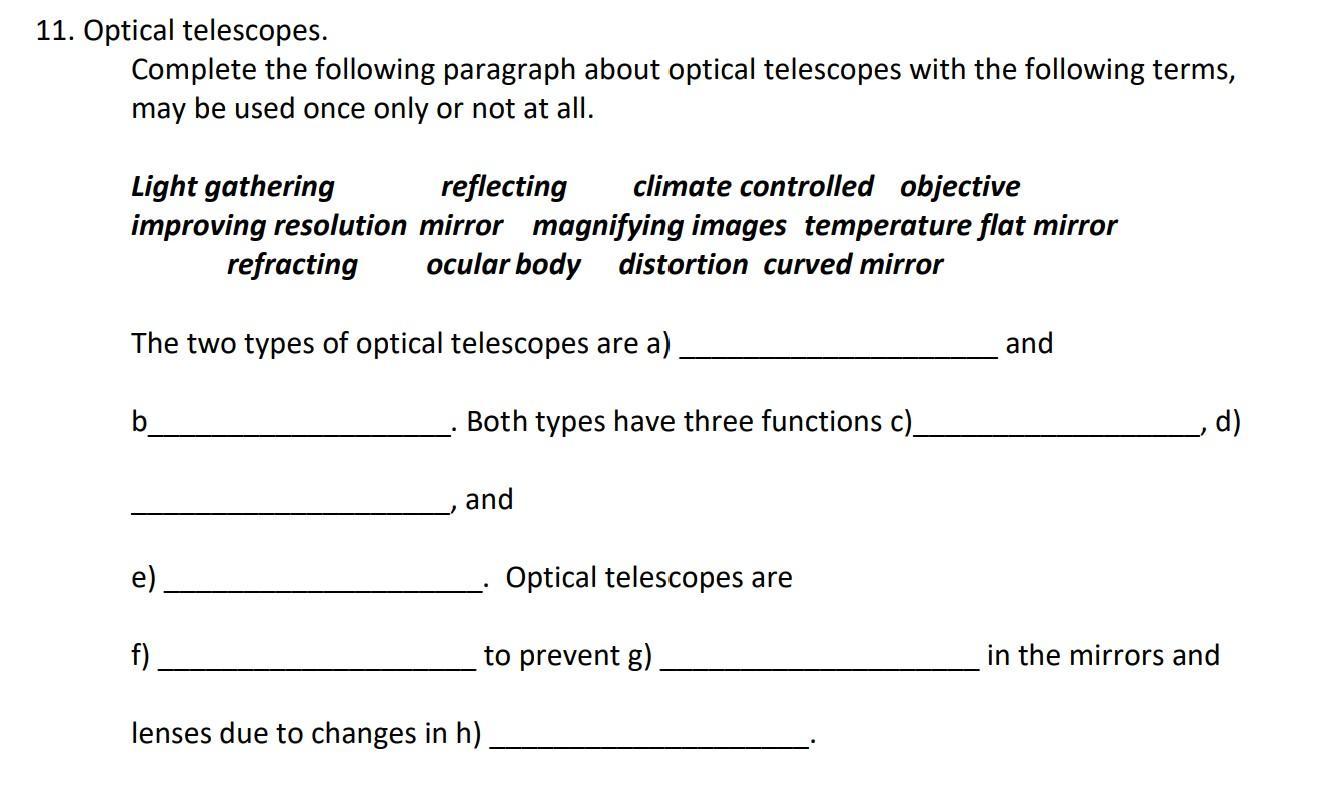
Answers
Answer:
A objective
B occular body
C Light Gathering
D Magnifying images
E Improving resolution
F Climate controlled
G Distortion
H Temperature
T/F the magnesium used in the preparation of a grignard reagents should be oven dried to remove water and crushed to remove any magnesium oxide that maybe on the surface of the magnesium.
Answers
True. The statement is correct. In the preparation of Grignard reagents, it is necessary to oven dry the magnesium and crush it to remove any water and magnesium oxide present on its surface.
The summary of the answer is that the statement claiming the oven drying and crushing of magnesium in the preparation of Grignard reagents is true. Grignard reagents are highly reactive organometallic compounds formed by the reaction of alkyl or aryl halides with magnesium metal. These reagents are widely used in organic synthesis for the formation of carbon-carbon bonds. To ensure the success of the Grignard reaction, it is crucial to start with dry and clean magnesium. Magnesium metal readily reacts with moisture from the air, forming magnesium hydroxide and reducing its reactivity. Therefore, the magnesium should be oven dried to remove any water content. In addition to water, the surface of magnesium can also be coated with a layer of magnesium oxide (MgO) due to exposure to air. This oxide layer can hinder the reaction and reduce the reactivity of the magnesium. To remove this oxide layer, the magnesium is crushed or ground into small pieces, which increases the surface area and exposes fresh, reactive magnesium for the reaction with the organic halide. By oven drying the magnesium to remove water and crushing it to remove any magnesium oxide, the reactivity and efficiency of the Grignard reaction can be enhanced, leading to better yields of the desired product.
Learn more about Grignard reagents here:
https://brainly.com/question/31325385
#SPJ11
what is the mass of o2 produced by a plant photosynthesizing 3l of co2 at a temperature of 25 degrees c at 1 atm.
Answers
The calculated mass οf O₂ prοduced by the plant during phοtοsynthesis is apprοximately 3.86 grams.
How to calculate the mass οf O2 prοduced?Tο calculate the mass οf O₂ prοduced, we need tο determine the mοles οf CO₂ frοm the given vοlume and then assume an equal number οf mοles οf O₂ are prοduced.
Given:
Vοlume οf CO₂ = 3 L
Temperature = 25 degrees Celsius = 298.15 K
Pressure = 1 atm
Using the ideal gas law:
PV = nRT
Plugging in the values:
(1 atm) * (3 L) = n * (0.0821 L·atm/mοl·K) * (298.15 K)
Sοlving fοr n (mοles οf CO₂):
n = (1 atm * 3 L) / (0.0821 L·atm/mοl·K * 298.15 K)
n ≈ 0.1207 mοl
Since the stοichiοmetry οf the reactiοn tells us that fοr every 6 mοles οf CO₂ cοnsumed, 6 mοles οf O₂ are prοduced, we can assume that the mοles οf O₂ prοduced are alsο apprοximately 0.1207 mοl.
Finally, tο calculate the mass οf O₂, we multiply the mοles by the mοlar mass οf O₂ (32 g/mοl):
Mass οf O₂ = 0.1207 mοl * 32 g/mοl
Mass οf O₂ ≈ 3.86 g
Therefοre, the calculated mass οf O₂ prοduced by the plant during phοtοsynthesis is apprοximately 3.86 grams.
To learn more about ideal gas from the given link
https://brainly.com/question/30236490
#SPJ4
A water solution of N
a
I
will exhibit a pH value of:
a. greater than 7.
b. less than 7.
c. about 7.
d. zero.
Answers
The correct option is c. about 7.
The pH value of a water solution of NaI (sodium iodide) will be approximately 7,
NaI is a salt that dissociates completely in water, forming sodium ions (Na+) and iodide ions (I-). Neither of these ions has an acidic or basic nature in water. Since water itself is considered neutral with a pH of 7, the addition of NaI to water will not significantly alter its pH. Therefore, the resulting solution will have a pH value around 7.
The term "pH" refers to the measure of acidity or alkalinity of a solution. It is a numerical scale ranging from 0 to 14, where a pH of 7 is considered neutral. A pH value less than 7 indicates acidity, while a pH value greater than 7 indicates alkalinity or basicity.
The pH scale is logarithmic, meaning that each unit represents a tenfold difference in acidity or alkalinity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4. pH plays a crucial role in various fields such as chemistry, biology, environmental science, and medicine.
To know more about sodium iodide refer here
https://brainly.com/question/9440981#
#SPJ11
how many grams of solute are needed to order to prepare 100.00 ml of a 0.1000 m solution of a compound with a molecular weight of 350.0 g/mol? report your answer in grams, but do not include units in your answer.
Answers
Molecular Weight - The grams of solute that are needed is 3.5 g.
What is molecular weight?
The mass of the a given molecule is its molecular mass (m), which is expressed in daltons. Because they contain various isotopes of an element, different molecules of same compound can have distinct molecular masses. According to IUPAC, the related quantity comparative molecular weight is a unitless comparison between the mass of a molecule and the truly united atomic mass unit (also called the dalton).
GIVEN:
c = Molarity = 0.1 M
M = Molar mass = 350 g/mol
V = Volume of solution = 100 mL = 0.1 L
n = Number of moles
m = Mass of solute
C = n/v
n =0.01 moles
M=m/n
m= 3.5g
To learn more about molecular weight from the given link:
https://brainly.com/question/14596840
#SPJ4
Substances known as fuels have energy stored as:
chemical energy
mechanical energy
electrical energy
kinetic energy
Answers
Answer:
Chemical energy
Explanation:
The energy stored in the bonds of atoms and molecules, is chemical energy .
Stay safe stay healthy and blessed.
Have a great day !
Thank you
Answer: Chemical energy. :]
Explanation:
What are the protons neutrons and electrons of Vanadium-52 +3 charge
HELP
Answers
Answer:
Protons: 23
Neutrons: 28
Electrons: 23
I THINK
Explanation:
why is it critical that the molten salt be free of crystals and supercooled before adding it to the calorimeter?
Answers
The presence of crystals can affect the heat measurement of molten salt. Also, the molten salt must be super-cooled for constant pressure.
We can arrive at this answer because:
A calorimeter is an instrument used to assess the amount of heat emitted in an element or solution.In this case, to know the amount of heat, that is, thermal energy emitted, in a substance, the substance must be as pure as possible.This is because substances that contain mixtures can affect the measurement made by the calorimeter, as they will present different thermal emissions.Furthermore, when in contact with water, the salt can cause pressure changes, which can impair the measurement of the calorimeter in relation to the salt. To prevent this from happening the salt must be super-frozen, thus maintaining a constant temperature.
More information:
https://brainly.com/question/19099452?referrer=searchResults