A soft lump of clay has water run on top of it. Most of the water and clay runs off the table. After a long while, the water is turned off and allowed to dry. There is no clay left; instead, there are small pebbles and other types of components left on the table.
Which natural process is this modeling?
Answers
The natural process being modeled is weathering, specifically physical weathering.
Physical weathering is the process by which rocks and minerals are broken down into smaller pieces without changing their chemical composition. Water is one of the most significant agents of physical weathering.
The scenario described in the question illustrates how water can cause physical weathering by soaking into a lump of clay, then drying out, leaving behind small pebbles and other components. The water expands as it freezes, causing the clay to crack, and as it dries, it evaporates, leaving behind the broken pieces.
Over time, this process can break down larger rocks and minerals into smaller particles, creating sediment that can be transported by wind, water, or ice, and deposited elsewhere. The result of physical weathering is often a mix of angular fragments that have the same composition as the original rock or mineral.
To know more about physical weathering, refer here:
https://brainly.com/question/29616084#
#SPJ11
Related Questions
briefly explain any two characteristics of photoelectric effect
Answers
2. The existence of a threshold frequency.
1. A gas at 24.0 Celsius and.900 atm fills a 0.95 Liter container.
What is its ending pressure (in atm) if the temperature changes
to 75 Celsius? Show all your work.
Answers
Answer:
P₂ = 1.05 atm
Explanation:
Given data:
Initial temperature = 24.0 °C (24+273 = 297 K)
Initial pressure = 0.900 atm
Final pressure = ?
Final temperature = 75 °C (75 + 273 =348 K)
Volume = constant
Solution:
According to Gay-Lussac Law,
The pressure of given amount of a gas is directly proportional to its temperature at constant volume and number of moles.
Mathematical relationship:
P₁/T₁ = P₂/T₂
Now we will put the values in formula:
0.900 atm / 297 K = P₂/348 K
P₂ = 0.900 atm × 348 K / 297 K
P₂ = 313.2 atm. K /297 K
P₂ = 1.05 atm
if you began with 2.3 g of 85% phosphoric acid, how many liters would this be?
Answers
According to the question 0.027647352941176 liters phosphoric acid would this be.
What is phosphoric acid?Phosphoric acid is an inorganic acid composed of phosphorus and oxygen, with the chemical formula H3PO4. It is an odorless, colorless, syrupy liquid that is non-flammable and slightly acidic. It is a tribasic acid, meaning that it has three ionizable hydrogen atoms, making it a strong acid when in aqueous solution. Phosphoric acid is used in many applications including food processing and production, pharmaceuticals, and various industrial processes.
2.3 g of 85% phosphoric acid is the same as 2.3 g of 85% H3PO4. To calculate the number of liters, we first need to convert the mass of H3PO4 to moles.
1 mole of H3PO4 has a mass of 98.00 g. Therefore, 2.3 g of H3PO4 is equal to 0.0235 moles.
We can now use the molarity formula to calculate the number of liters:
Molarity = moles/liters
liters = moles/Molarity
liters = 0.0235 moles/0.85
liters = 0.027647352941176 liters
To learn more about phosphoric acid
https://brainly.com/question/14812997
#SPJ4
a noncovalent interaction between two molecules is known as
Answers
A non-covalent interactions between molecules are van der Waal's forces and hydrogen bonds.
What are non-covalent interactions?
Non-covalent interaction is an interaction which does not involve sharing of electrons and in this aspect it differs from covalent bond.It rather involves dispersed variations of electromagnetic interactions which are present between the molecules or within the molecule.
The energy released during the formation of these interactions is of the order of 1-5 kcal .They are classified as electrostatic, pi effects , van der waals forces and hydrophobic effects.
They are important in maintaining the three dimensional structure of large molecules such as proteins ,nucleic acids ,etc.They are also involved in biological processes . They heavily influence drug design process and design of materials.
Learn more about non-covalent interactions,here:
https://brainly.com/question/10736477
#SPJ2
Which geologic features help scientists determine the relative ages of rocks by their positions? Select three options.
magma
erosion
intrusions
index fossils
cross-cutting relationships
helppp i need it nowwwww
Answers
Answer:
Option D, Index fossil helps scientists to determine the relative ages of rocks by their positions
Explanation:
Index fossils are arranged in layer in such a way that the lowest layer represent the oldest fossil and the top most layer represents the youngest fossil. Scientist use this concept to determine the relative age of the rocks based on their position beneath the earth’s surface
Hence, option D is correct
Can someone do a True or false for these

Answers
Answer:
all i can accurately say is that 2 and 4 are both true
In order to assess the spontaneity of a chemical reaction or physical process both the change in ________ and ________ associated with the reactions process must be known
Answers
In order to assess the spontaneity of a chemical reaction or physical process, both the change in enthalpy (ΔH) and entropy (ΔS) associated with the reaction or process must be known.
The summary of the answer is that to determine if a chemical reaction or physical process is spontaneous, we need to consider the changes in both enthalpy and entropy. Enthalpy (ΔH) refers to the heat energy exchanged during a reaction or process. It represents the difference between the energy of the products and the energy of the reactants. A negative ΔH indicates an exothermic reaction or process, where heat is released, while a positive ΔH indicates an endothermic reaction or process, where heat is absorbed. Entropy (ΔS) is a measure of the disorder or randomness in a system. It represents the change in the number of energetically equivalent microstates available to the system. An increase in entropy (positive ΔS) means an increase in disorder, while a decrease in entropy (negative ΔS) means a decrease in disorder. For a reaction or process to be spontaneous, it generally requires a decrease in enthalpy (ΔH < 0) and/or an increase in entropy (ΔS > 0). This can be determined by evaluating the Gibbs free energy change (ΔG), which combines the effects of enthalpy and entropy (ΔG = ΔH - TΔS, where T is the temperature in Kelvin). If ΔG is negative, the reaction or process is spontaneous. If ΔG is positive, the reaction or process is non-spontaneous.
Learn more about Enthalpy here:
https://brainly.com/question/21369686
#SPJ11
The law of conservation of mass is applicable to
Answers
Answer:
The law of conservation of mass states that in a closed system, mass is neither created nor destroyed during a chemical or physical reaction. The law of conservation of mass is applied whenever you balance a chemical equation.
Explanation:
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
It is applicable in a chemical when the the mass of the products in a chemical reaction is equal to the mass of the reactants.
But it is not applicable in a nuclear fusion as some of the mass is generated as energy.
What is the principal positively charged ion inside body cells?
a. Calcium
b. Sodium
c. Magnesium
d. Potassium
e. Chloride
Answers
The principal positively charged ion which is inside body cells is potassium (K+).
Option D is correct.
Potassium ions play a crucial role in various physiological processes, including maintaining the resting membrane potential of cells, which is essential for proper nerve impulse transmission and muscle function. While other ions like sodium (Na+), calcium (Ca2+), and magnesium (Mg2+) also have important roles in cellular function, potassium is the predominant positively charged ion found inside cells.
Therefore, the correct answer is d. Potassium.
Learn more about Potassium:
brainly.com/question/24527005
#SPJ11
MARKING BRAINLIEST!! - Determine the molar mass [MM] of a gas if 2 L of the gas weights 0.500 g at 298 K and 2.00 atm.
Answers
Answer:
3.125g/mol
Explanation:
To find the molar mass of the gas, we need to initially find the number of moles (n) contained in the gas. To find the number of moles, we use the general gas law whose equation is:
PV=nRT
Where; P= Pressure
V= Volume occupied by gas
n= number of moles
R= general gas constant
(0.0821 L atm mol/K)
T= absolute temperature
According to the question; P= 2.0atm, V= 2.0L, n= ?, T= 298K
To find n, we make it the subject of the formula:
n= PV/RT
n= 2.0 × 2.0 / 0.0821 × 298
n= 4/ 24.4658
n= 0.16mol
If number of moles (n) of the gas is 0.16mol and it weighs 0.500g, its molar mass can be found using:
number of moles (n) = mass (g) / molar mass
Making MM subject of the formula;
molar mass = mass / number of moles
MM= 0.500/0.16
MM= 3.125
Hence, the molar mass of the gas is 3.125g/mol.
Which of the following is NOT an organic compound?

Answers
Answer:
the first one
Explanation:
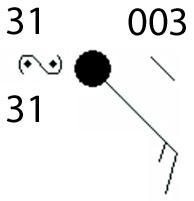
The picture below shows a weather station model at a location.
What type of weather is expected at the location?
A. fog
B. haze
C. dust storm
D. freezing rain
Answers
The type of weather expected according to the symbol is freezing rain. Option D
How can you identify the weather in the picture?The weather symbol for freezing rain is a capital S with one or two points.
The points represent the intensity of the freezing rain. One point indicates light freezing rain, while two points indicate moderate freezing rain.
Freezing rain is known as a type of precipitation that occurs when rain falls through a layer of cold air and freezes on contact with the ground or other surfaces.
It can cause dangerous driving conditions, power outages, and other disruptions.
Find more exercises on weather symbols;
https://brainly.com/question/29559036
#SPJ1
a weak base has kb=3.2×10−6. the compound has a solubility of 1.667gl, and the molar mass is 303.36gmol. what is the ph of a saturated solution of this compound?
Answers
The pH of the saturated solution of the weak base is approximately 11.08.
To determine the pH of a saturated solution of the weak base with the given information, we can first find the concentration of the base in moles per liter.
1. Calculate the concentration of the weak base:
Solubility = 1.667 g/L
Molar mass = 303.36 g/mol
Concentration = (1.667 g/L) / (303.36 g/mol) = 0.0055 mol/L
2. Use the Kb value to find the concentration of OH- ions:
Kb = [OH-][HB+] / [B]
Kb = 3.2 × 10^-6
Since the weak base partially dissociates in water, we can assume that [OH-] = [HB+] and [B] ≈ 0.0055 mol/L. Therefore:
Kb = (x)(x) / (0.0055 - x) ≈ x^2 / 0.0055
Solve for x (concentration of OH-):
x = √(Kb * 0.0055) = √(3.2 × 10^-6 * 0.0055) = 0.0012 mol/L
3. Calculate the pOH and pH:
pOH = -log[OH-] = -log(0.0012) = 2.92
pH = 14 - pOH = 14 - 2.92 = 11.08
The pH of the saturated solution of the weak base is approximately 11.08.
Learn more about saturated solution here
https://brainly.com/question/1851822
#SPJ11
Help, please!
Which property of a substance can be determined using a pH indicator ?
Density
Acidity
Electrical Conductivity
Thermal Conductivity
Boiling Point

Answers
How many grams of oxygen is present in 36g of water?
Answers
Answer:
32 gm O
Explanation:
there are 16 gm of O in each 18 gm of water ....the other two grams are H
so there would be 32 gm of oxygen in 36 gm water
what is the transformation efficiency of e coli hb101 when using the claciu chloride
Answers
The transformation efficiency of E. coli HB101 using calcium chloride can vary depending on the experimental conditions and the protocol used.
However, transformation efficiency is a measure of how many bacterial cells take up the foreign DNA and become genetically transformed, usually reported as the number of transformants per microgram of DNA. To calculate the transformation efficiency of E. coli HB101 using calcium chloride, follow the 5 steps:
1. Perform a transformation experiment using E. coli HB101 and calcium chloride. This usually involves treating the bacterial cells with calcium chloride to make them more permeable to foreign DNA, then exposing them to the DNA of interest.
2. Plate the transformed cells on selective agar plates that will allow only the transformed cells to grow.
3. Count the number of transformant colonies that appear on the selective agar plates after a suitable incubation period.
4. Determine the amount of DNA (in micrograms) used in the transformation experiment.
5. Calculate the transformation efficiency by dividing the number of transformant colonies by the amount of DNA used.
The result will be in the unit of transformants per microgram of DNA. Keep in mind that the transformation efficiency can be influenced by factors such as the quality and concentration of the DNA, the bacterial strain, and the specific experimental conditions. Therefore, the transformation efficiency for E. coli HB101 using calcium chloride may differ between experiments and laboratories.
Learn more about calcium chloride at brainly.com/question/664620
#SPJ11
write an equation representing each of the following: (a) the change of a potassium atom to a potassium ion
Answers
The equation K → K+ + e- represents the ionization of a potassium atom through the loss of one electron.
What does the equation K → K+ + e- represent?
The equation for the change of a potassium atom to a potassium ion can be written as:
K → K+ + e-
This equation represents the loss of one electron (e-) by a neutral potassium atom (K) to form a positively charged potassium ion (K+).
The equation K → K+ + e- represents the transformation of a neutral potassium atom (K) to a positively charged potassium ion (K+) through the loss of one electron (e-). When an atom loses or gains electrons, it becomes an ion with a net electrical charge. In this case, the potassium ion has a positive charge since it has lost one electron. This process is called ionization and is important in many chemical reactions, including those that occur in the human body. Potassium ions, for example, are essential for nerve impulse transmission and muscle contraction.
To learn more about ionization, visit: https://brainly.com/question/30831422
#SPJ4
Cocoa beans are subjected to three processes during the manufacture of chocolate: cleaning, roasting, and 'nibbing'. Bags of cocoa beans are first cleaned, then cleaned beans are roasted, then roasted
Answers
Beans are processed through 'nibbing'. During the nibbing process, the roasted cocoa beans are crushed and ground into a paste called cocoa mass or cocoa liquor.
This cocoa mass can then be further processed to separate the cocoa solids from the cocoa butter, which is the fat component of the cocoa bean. The separated cocoa solids and cocoa butter are used in the production of chocolate. Pure cocoa mass (cocoa paste) in solid or semi-solid form is known as chocolate liquor. It includes about equal amounts of cocoa butter and solid cocoa, much like the cocoa beans (nibs) from which it is made. It is made from fermented, dried, roasted, and separated from their skins cocoa beans. To make cocoa mass (cocoa paste), the beans are pulverised.
To know more about cocoa liquor
https://brainly.com/question/5047676
#SPJ11
e a balanced half-reaction for the reduction of gaseous oxygen to aqueous hydrogen peroxide in acidic aqueous solution. be sure to add physical state symbols where appropriate.
Answers
The balanced half-reaction of the reduction of the gaseous oxygen to the aqueous hydrogen peroxide in the medium of the acidic aqueous solution.
O₂(g) + 2H⁺ + e⁻ ----> H₂O₂(aq)
This chemical equation is as :
O₂(g) ----> H₂O₂(aq)
The half reaction in which the reduction will takes place :
O₂(g) ----> H₂O₂(aq)
The Hydrogen atoms for the half reduction reaction will be balanced by the addition of the six hydrogen atoms are :
O₂(g) + 2H⁺ ----> H₂O₂(aq)
The charge will be balance in the half reaction, we get the balance equation is :
O₂(g) + 2H⁺ + e⁻ ----> H₂O₂(aq)
To learn more about half-reaction here
https://brainly.com/question/18403544
#SPJ4
T/F All amino acid based hormones are lipid soluble and can cross the plasma membrane.
Answers
False All the amino acid based hormones are not lipid soluble and can not cross the plasma membrane.
Amino acid-derived as well as polypeptide hormones are water-soluble and insoluble in lipids. These hormones cannot pass through the plasma membranes of the cells; therefore, their receptors are found on the surface of the target cells.
Amino acids are those molecules which will combine to form proteins. When the proteins are digested or broken down, amino acids will be left. The human body uses amino acids to make proteins to help out the body: Repair body tissue, Grow, and Perform many other body functions.
Amino acids are also be used as a source of energy by the body.
To know more about amino acid here
https://brainly.com/question/15823799
#SPJ4
??????????????????????????????????????????
Answers
Answer:
??????????????????????????????‽??????????‽??‽?‽
help me with this questions please! i would like a answer for both if thats okay thank you so much!

Answers
Answer: 1. Gravity is very important to us. We could not live on Earth without it. The sun's gravity keeps Earth in orbit around it, keeping us at a comfortable distance to enjoy the sun's light and warmth. It holds down our atmosphere and the air we need to breathe. Gravity is what holds our world together.
2. Just like objects and people, Earth is also attracted by the sun’s gravity. If Earth’s gravity became zero, nothing would hold it and chances are that its inner core would eventually burst in a lethal titanic explosion due to intense pressure. Earth would break into pieces that would float around space, wreaking havoc.
Explanation:
if 5.4 moles of Na₂CO₃ react with excess calcium hydroxide. how many grams of CaCO₃ will be produced?
Na₂CO₃+Ca(OH)₂=2NaOH+CaCO₃
Answers
Answer:
540.47g approximately
Explanation:
No. of moles in Na₂CO₃ = 5.4 moles
Mole ratio of Na₂CO₃ : CaCO₃ = 1:1
No. of moles in CaCO₃ = 5.4 moles
Mass of CaCO₃ = 5.4 × 100.0869
= 540.46926g
Select all the correct answers.
Which of the following are considered pure substances?
element
compound
homogeneous mixture
heterogeneous mixture
Answers
Element and compound both are considered as pure substances.
Thus, both option 1 and 2 are correct.
What is a pure substance?Pure substances are defined as materials with a consistent chemical composition that cannot be physically separated through the use of techniques like filtration, evaporation, etc.
Anything that is made up of the same atom or molecule throughout is a pure substance. Everything else is a mixture.
Since elements are not physically or chemically broken down to generate simple, tiny entities like zinc, elements and compounds are pure substances. Furthermore, pure substances like water are combined to create compounds.
Both homogeneous and heterogeneous mixtures are not pure substances since they lack predetermined ratios and proportions, making it difficult to identify their exact composition.
Pure substances include sodium, water, a copper wire, an aluminum foil, etc.
Thus, elements and compounds both are categorised as pure substances.
To learn more about pure substances, refer to the below link:
https://brainly.com/question/11134780
#SPJ2
which type of electronic radiation has a lower frequency than infrared radiation? A gamma rays B microwave C visible light D x-ray
Answers
Answer:
A, gamma rays
Explanation:
Radio waves, on the other hand, have the lowest energies, longest wavelengths, and lowest frequencies of any type of EM radiation. In order from highest to lowest energy, the sections of the EM spectrum are named: gamma rays, X-rays, ultraviolet radiation, visible light, infrared radiation, and radio waves.
when an action potential abides by the all-or-nothing principle, once it reaches its threshold it moves all the way down the axon
Answers
Answer:
Yep and it's threshold is -55 mV.
What is the v if = 8 m and f = 20 Hz?
Answers
Answer:
Explanation:
it is 980
question 1 a spreadsheet cell contains the coldest temperature ever recorded in new zealand: -22 °celsius. what function will display that temperature in fahrenheit?
Answers
When the temperature conversion function =CONVERT(-22, "C", "F") is applied, a reading of -22 °C in Fahrenheit is displayed. On a variety of scales, including the Fahrenheit and Celsius systems, temperature is a unit that is used to denote hotness or coolness.
Heat energy will logically go from a hotter (body with a higher temperature) to a colder (body with a lower temperature) according to temperature (one at a lower temperature).
A temperature is a measurement used to express how hot or cold something is. It demonstrates how heat energy naturally flows from a hotter body to a cooler body and can be expressed in terms of any number of arbitrary scales (one at a lower temperature).
A match is burning at a far greater temperature than an iceberg, yet an iceberg has a significantly higher total heat energy than a match. Temperature is not the same as the energy of a thermodynamic system.
The temperature, along with pressure, density, and other similar properties, is referred to as an intense property as opposed to extensive characteristics like mass or volume—one that is independent of the quantity of stuff being addressed.
To know more about temperature:
brainly.com/question/23411503
#SPJ4
Which event most likely occurs at point v? cooling erosion heating melting
Answers
The event which most likely occurs at point V during a rock cycle is cooling.
What is point V?Point V is present in the rock cycle, where changes in the state or nature of rock takes place.
When mangma comes towards the surface of the earth then due to change in temperature and pressure it will concerts into the igneous rock by cooling process and coverts into the harder form.
Hence at point V, colling is occur.
To know more about rock cycle, visit the below link:
https://brainly.com/question/6073070
Answer:
A. cooling
Explanation:
edge 22'
what is the molality of a solution made by dissolving 36.5 g of naphthalene 1c10h82 in 425 g of toluene 1c7h82?
Answers
The molality of a solution made by dissolving 36.5 g of naphthalene C₁₀H₈ in 425 g of toluene C₇H₈ is 0.67 m.
The molality of a solution is calculated by dividing the moles of solute by the mass of solvent in kilograms. The formula for molality is:
molality = moles of solute / mass of solvent (kg)
First, we need to calculate the moles of naphthalene (C₁₀H₈):
moles of naphthalene = mass of naphthalene / molar mass of naphthalene
moles of naphthalene = 36.5 g / 128.17 g/mol
moles of naphthalene = 0.2846 mol
Next, we need to convert the mass of toluene (C₇H₈) from grams to kilograms:
mass of toluene = 425 g / 1000
mass of toluene = 0.425 kg
Finally, we can calculate the molality of the solution:
molality = 0.2846 mol / 0.425 kg
molality = 0.6696 mol/kg
Therefore, the molality of the solution is 0.67 mol/kg or 0.67m.
Learn more about molality here: https://brainly.com/question/24065939.
#SPJ11