An unidentified gas a density of 2. 40 g/L when measured at 45°C and 820 torr pressure. Calculate
the molar mass of this gas
Answers
The molar mass of the unidentified gas is 40.06 g/mol.
To calculate the molar mass of the gas, we can use the ideal gas law, PV=nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
We can rearrange this equation to solve for the number of moles:
n = PV/RT
We can then use the definition of density, d = m/V, where m is the mass, to solve for the mass of the gas:
m = dV
We can substitute these expressions into the equation for n:
n = (dV)P/RT
We can then use the definition of molar mass, M = m/n, to solve for the molar mass:
M = m/n = (dV)P/RT
Substituting the given values, we have:
M = (2.40 g/L)(0.820 atm)(22.4 L/mol)/(0.0821 L·atm/mol·K)(318 K) = 40.06 g/mol
To know more about molar mass, refer here:
https://brainly.com/question/22997914#
#SPJ11
Related Questions
Do the follow reactions depict heat of formation reactions? if so, mark the reaction as yes, if it is not a heat of formation reaction, then choose no.
Answers
The amount of heat created or absorbed during a reaction that takes place under a constant amount of pressure is measured by the enthalpy change.
What causes formation heat to be produced?The change in enthalpy that occurs as one mole of a compound is created from its component parts is known as the "heat of formation." The total amount of energy that should be added to or released during a chemical reaction is known as the heat of reaction.
What signs do you see when heat is produced?By deducting the total of the standard enthalpies of formation of the reactants from the sum of the standard enthalpies of formation of the reactants and products, one can determine the standard enthalpy change of formation.
To know more about Heat of formation visit:
https://brainly.com/question/13096727
#SPJ4
Determine whether each of the following reactions is spontaneous.
AHsystem = -75.9 kJ, T = 273 K,
ASsystem = 138 JIK
Answers
Answer:
gxibdkkzvsosvjzkxvdkbdsibsisbdkbslabdldvslbskskd
Jane and Jack have a bicycling competition Jane rides a constant speed of 12 miles per hour while Jack rides at a constant speed of 330 feet per minute. How fast are Jane and Jack going in meters per second? Who finishes first? Show your factor-label method work. There are 5280 feet in one mile. There are 3.281 feet in one meter
Answers
Answer:
Explanation:
I don't know how you want the conversion done. I use dimensional analysis.
Jane
12 miles / hour [1.6 km/1 mile][1000 m/1 km][1 hour / 3600 sec]
12 * [1.6 * 3600 / 1000 m/s] = 69.12 m/s
5.33 m/s This answer is a bit shorter than using 5280 feet.
Using 5280 feet
12 miles / hour [5280 ft/1 mile] [1 m/3.281 feet] * [1 hr/3600 sec]
12 * 5280 / (3.281 * 3600)
12 *. 4470
5.36
Jack
330 feet / minute [ 1 meter / 3.281 feet] [1 minute / 60 seconds]
330 * 1/(3.281 * 60)
330 * 1/(196.86)
1.676 m/s
She's going faster than he is, no matter which method is used to do the calculation
How many formula units of potassium
sulfide are present in 225 g K2S?
225 g
6.02 x 1023 fun
1 mole
110.26 g
1 mole
[?] × 10²] fun
Answers
Answer:
1.23 x 10²⁴ formula units K₂S
Explanation:
To find the amount of formula units in potassium sulfide, you need to (1) convert grams to moles (via the molar mass) and then (2) convert moles to formula units (via Avogadro's number). The conversions/ratios need to be arranged in a particular way that allows for the cancellation of units. Units are cancelled when then are located both in the numerator and denominator somewhere in the math. The final answer should have 3 sig figs because the given value (225 grams) has 3 sig figs.
Avogadro's number:
6.02 x 10²³ formula units = 1 mole
Molar Mass of K₂S:
110.26 g/mol
225 g K₂S 1 mole 6.02 x 10²³ units
---------------- x ----------------- x ---------------------------- = 1.23 x 10²⁴ formula units
110.26 g 1 mole
Explain the concept law of diminishing marginal rate of substitution. What is/are the reason/s why the law of diminishing marginal rate of substitution suggest/s that isoquant must be bent toward the origin?
Answers
The law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
In other words, as the quantity of one good increases, the individual is willing to sacrifice fewer units of the other good to obtain an additional unit of the first good. This reflects a diminishing rate of substitution between the two goods.
The reason why the law of diminishing marginal rate of substitution suggests that isoquants must be bent toward the origin is rooted in the concept of diminishing marginal utility. As more units of a particular input (e.g., labor or capital) are added while holding other inputs constant, the additional output gained from each additional unit of the input will decrease. This diminishing marginal productivity leads to a decreasing MRS.
When isoquants (which represent different combinations of inputs that produce the same level of output) are bent toward the origin, it reflects the fact that as more of one input is used, the amount of the other input that needs to be substituted decreases. This bending signifies the diminishing MRS and captures the idea that a larger quantity of one input can be substituted for a smaller quantity of the other input to maintain the same level of output.
Overall, the law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
To know more about marginal rate of substitution, click here, https://brainly.com/question/30763866
#SPJ11
I need help in this question pleaaaaase!
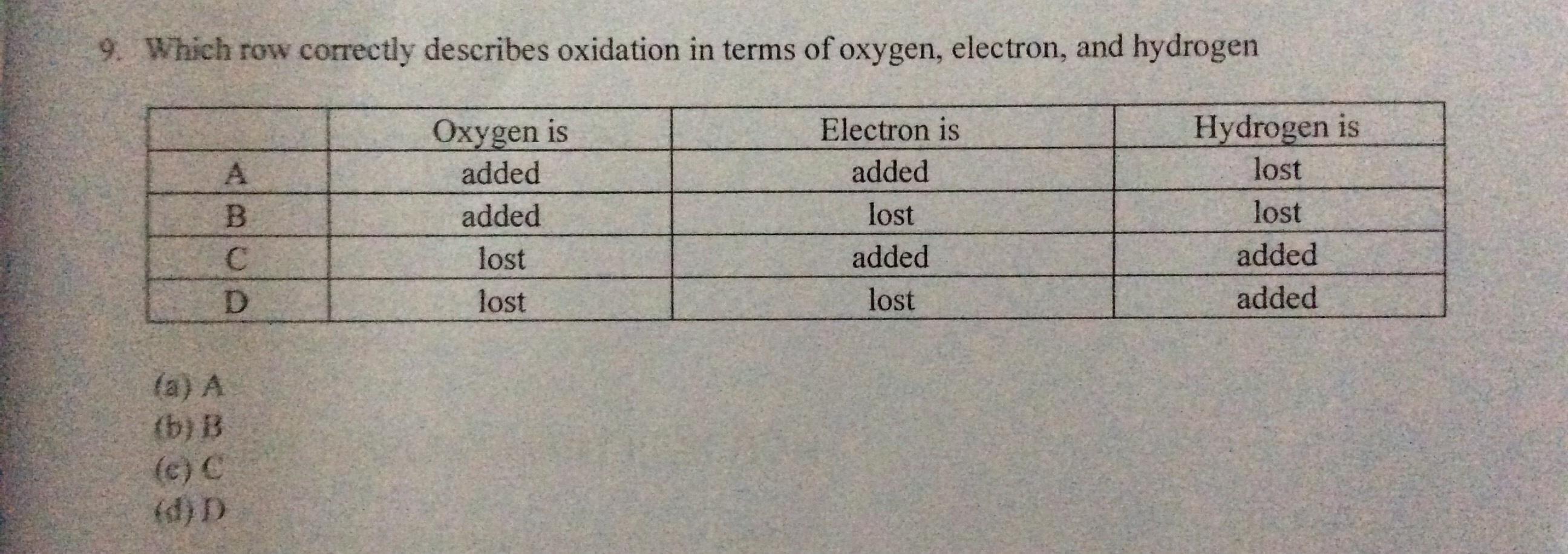
Answers
The correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
What is an oxidation reaction?An oxidation reaction is a reaction in which the oxidation number if the reacting species increases in a positive direction.
Oxidation can be defined in terms of oxygen as the addition of oxygen. Oxidation can be defined in terms of electron as removal of electron Oxidation can be defined in terms of hydrogen as removal of hydrogen.Therefore, the correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
Learn more about oxidation at: https://brainly.com/question/4222605
What does the reducing agent do in a redox reaction? (A.P.E.X)
Answers
Answer:
The reducing agent donates electron to the oxidizing agent and gets oxidized itself
Explanation:
In an oxidation-reduction reaction, also known as REDOX reaction, one of the reactants is called OXIDIZING AGENT while the other is called REDUCING AGENT. The reducing agent is called so because it reduces another substance in the reaction.
It reduces another atom/ion by donating electrons to that atom, hence, getting oxidized itself in the process. For example, in the following reaction:
H2 + F2 → 2HF
Fluorine is getting oxidized from -2 to 0 by donating electrons to H and reducing it from +2 to 0.
Answer:
Reduces another atom
Explanation:
:)
How many molecules are the in 1.8g of water molecules
Answers
Answer:
6.022 x \(10^{22}\) molecules
Explanation:
One mole of any substance has 6.022x \(10^{23}\) particles.
Mr of water: 16 + 2 = 18 g
moles of water given: 1.8/18 = 0.1 moles
Using ratio:
moles : molecules
1 : 6.022x \(10^{23}\)
0.1 : X
X = 0.1 x (6.022x \(10^{23}\) )
X = 6.022 x \(10^{22}\)
A force of 20N acts on an object. It causes a displacement of 500 cm in the direction of force. What would be the work done in Nm on the object?
Answers
Answer:
500/100=5m
W=F*D
W=20*5=100Nm
Explanation:
The work done in Nm on the object is 100 Nm
Calculation of the work done:
Since A force of 20N acts on an object. It causes a displacement of 500 cm in the direction of the force.
Since the displacement is in cm
So, in meters, it should be like
= 500 / 100
= 5 m
Now we know that
Work done = force * displacement
= 20N * 5
= 100 Nm
hence, The work done in Nm on the object is 100 Nm
Learn more about force here: https://brainly.com/question/21535944
Me podrían decir los nombres comunes de los siguientes hidrocarburos!! URGENTE AYUDA

Answers
Answer:
fdhhdhdhdhhshdgdvdvebehbehdhehdhhddhdf
Answer:
No Se Espanol
Explanation:
2. a. In general, how do the periodic properties of
the d-block elements compare with those of
the main-group elements?
b. Explain the comparison made in (a).
Answers
The d-block elements are transition metals. They still exhibit the properties expected of metals just like the main group elements even though they are not as reactive as the main group metals.
The periodic table is divided into blocks; s- block, p- block, d - block and f - block.
The s - block and p - block elements are called the main group elements. The elements of the d - block has some unique properties due to the presence of partially filled d - orbitals.
However, the d - block elements are metals and also undergo many of the reactions expected of metals. They still displace hydrogen from dilute acid and react with water(just a few of them) under appropriate conditions to liberate hydrogen gas etc.
However, the first row transition elements are more reactive than the second and third row which tends to be more noble.
Generally, the d - block elements are far less reactive than s - block and p - block metals.
Learn more: https://brainly.com/question/1948991
Answer:
The d-block elements are transition metals. They still exhibit the expected properties of metals but aren't as reactive .
The d-block is not part of the main group elements: s and p blocks. The d-block has unique properties due to the presence of partially filled d-orbitals.
Given:
CH4 + 2O2 → CO2 + 2H2O, ΔH = -890 kJ/mol
How much energy is released when 59.7 grams of methane (CH4) reacts with oxygen?
The combustion of 59.7 grams of methane releases ____ kilojoules of energy
Answers
Answer:
The combustion of 59.7 grams of methane releases 3320.81 kilojoules of energy
Explanation:
Given;
CH₄ + 2O₂ → CO₂ + 2H₂O, ΔH = -890 kJ/mol
From the combustion reaction above, it can be observed that;
1 mole of methane (CH₄) released 890 kilojoules of energy.
Now, we convert 59.7 grams of methane to moles
CH₄ = 12 + (1x4) = 16 g/mol
59.7 g of CH₄ \(= \frac{59.7}{16} = 3.73125 \ moles\)
1 mole of methane (CH₄) released 890 kilojoules of energy
3.73125 moles of methane (CH₄) will release ?
= 3.73125 moles x -890 kJ/mol
= -3320.81 kJ
Therefore, the combustion of 59.7 grams of methane releases 3320.81 kilojoules of energy
Answer:
The combustion will release -3,321 KJ/mol of energy
Explanation:
Iupac name for this?

Answers
Answer:
Explanation:
4,5 diethyl-2 fluoro-3-methylheptanal
Potassium iodide and lead(II) nitrate solutions react together to form a precipitate of lead(II) iodide: 2KI(aq) + Pb(NO3)2(aq) → PbI2(s) + 2KNO3(aq) In each of the following cases, carry out the calculations to determine the quantities required. a If 1.0 mol of potassium iodide reacts with 1.0 mol of lead(II) nitrate, determine which reactant is in excess and by how many moles. b If 0.50 mol of potassium iodide reacts with 2.0 mol of lead(II) nitrate, determine which reactant is in excess and by how many moles. c If 1.00 g of lead(II) nitrate reacts with 1.50 g of potassium iodide, determine which reactant is in excess and the mass of lead(II) iodide that forms. d If 50.0 mL of 1.00 M lead(II) nitrate solution reacts with 75.0 mL of 0.500 M potassium iodide solution, determine which reactant is in excess the mass of lead(II) iodide that forms
Answers
As a result, 0.231liters of the 0.150M potassium iodide solution would be required to thoroughly react with the supplied lead(II) nitrate solution.
What occurs when a solution of lead nitrate combines with a solution of potassium iodide?When a solution of potassium iodide is introduced to a solution of lead nitrate in a test tube, a yellowish solid precipitates.
Lead iodide is a whitish solid. Along with lead iodide, potassium nitrate is generated. This is a reaction with two displacements.
The reaction between lead nitrate [Pb(NO3)2] and potassium iodide (Kl) results in the creation of potassium nitrate (KNO3) and a yellow precipitate of lead iodide (PbI2).
Learn more about potassium iodide refer
https://brainly.com/question/2913015
#SPJ1
Suggest the pH of Calamine lotion.
Answers
Explanation:
Calamine solution contains zinc carbonate and is basic in nature. When applied in the skin, it reacts with the acid, and neutralizes its effect, thus giving relief.
If ∆Hº for a particular reaction is positive, select all the statements that correctly describe the reaction.
a) The reaction is endothermic
b) There is a net increase in the enthalpy of the system
c) More energy is needed to break bonds than is released by forming bonds
d) The bonds formed in the product(s) are stronger than the bonds broken in the starting material.
Answers
If \(HA^{0}\) for a particular reaction is positive, it indicates that the reaction is endothermic, meaning that heat energy is absorbed from the surroundings. This leads to a net increase in the enthalpy of the system.
This can also be understood in terms of bond strength - if the bonds formed in the product(s) were weaker than the bonds broken in the starting material, energy would be released, and \(HA^{0}\) would be negative. However, since the bonds formed in the product(s) are stronger than the bonds broken in the starting material, more energy is required to break these bonds and form the new product(s). Therefore, statements a) and b) are correct, while c) and d) are incorrect in this context.
In other words, if ΔH° for a particular reaction is positive, the correct statements that describe the reaction are: a) The reaction is endothermic. A positive ΔH° value indicates that the reaction absorbs energy from its surroundings, making it an endothermic reaction. b) There is a net increase in the enthalpy of the system. A positive ΔH° means that the enthalpy of the products is greater than the enthalpy of the reactants, resulting in a net increase in the system's enthalpy. c) More energy is needed to break bonds than is released by forming bonds. In a reaction with a positive ΔH°, the energy required to break the bonds in the reactants is higher than the energy released when new bonds are formed in the products. This leads to an overall energy absorption in the reaction.
Learn more about endothermic here :
https://brainly.com/question/23184814
#SPJ11
Carbon tetrachloride (CCIA) is: covalent or Ionic?
Answers
Answer:
covalent
Explanation:
How many moles of sodium hydroxide would react with 1 Mole of sulphuric acid?
Answers
Answer:
Two moles.
Explanation:
Sulphuric (sulfuric) acid \(\rm H_2SO_4\) is a diprotic acid. When one mole of \(\rm H_2SO_4\) molecules dissolve in water, two moles of \(\rm H^{+}\) ions would be produced.
\(\rm H_2SO_4 \to 2\, H^{+} + {SO_4}^{2-}\).
On the other hand, sodium hydroxide \(\rm NaOH\) is a monoprotic base. When one mole of \(\rm NaOH\) formula units dissolve in water, only one mole of hydroxide ions \(\rm OH^{-}\) would be produced.
\(\rm NaOH \to Na^{+} + OH^{-}\).
Note that \(\rm H^{+}\) and \(\rm OH^{-}\) react at a one-to-one ratio:
\(\rm H^{+} + OH^{-} \to H_2O\).
As a result, it would take \(2\; \rm mol\) of \(\rm OH^{-}\) to react with the \(\rm 2\; mol\) of \(\rm H^{+}\) that was released when \(1\; \rm mol\) of \(\rm H_2SO_4\) is dissolved in water. Since one mole of \(\rm NaOH\) formula units could produce only one mole of \(\rm OH^{-}\), it would take \(\rm 2\; mol\) of \(\rm NaOH\) formula units to produce that \(2\; \rm mol\) of \(\rm OH^{-}\) for reacting with \(1\; \rm mol\) of \(\rm H_2SO_4\).
Learning about the parts of an atom is important because
It can help us create a better model of an atom.
It can help us create new elements.
It can help us study how cells in the body functions.
It can help us explain the universe.
Answers
Answer:
"it can help us explain the universe" or "it can help us study how cells in the body function"
Explanation:
you choose which one you want but their still both technicly right
what is antidote for magnesium sulfate
Answers
The treatment for magnesium poisoning involves slowly injecting 10mL of calcium gluconate (2.2mmol of calcium in a 10mL vial; formerly known as 10% solution) into the vein over the course of 10 minutes.
What are the symptoms and treatment for magnesium sulfate toxicity?
Early symptoms of magnesium toxicity include nausea, feeling heated, flushing, somnolence, double vision, slurred speech, and weakness. The first sign of magnesium toxicity is typically the loss of patellar reflexes, which happens with serum magnesium levels of 9 mg/dl to 12 mg/dl.
What symptoms indicate toxicity from magnesium sulfate?
Keep an eye out for the warning signs and symptoms of magnesium sulfate toxicity, such as hypotension, areflexia (loss of DTRs), respiratory depression and arrest, oliguria, shortness of breath, chest pains, slurred speech, hypothermia, confusion, and circulatory collapse.
To know more about magnesium visit:
https://brainly.com/question/8732513
#SPJ1
when a sample of 89.56 g nh4no3 dissolves in 42.05 g of water, the temperature changes from 28.69 c to 7.8 c. calculate the heat flow (q)
Answers
When a sample of 89.56 g NH4NO3 dissolves in 42.05 g of water, the temperature changes from 28.69 c to 7.8 c, the heat flow (q) in this case is approximately -11,165 Joules.
To calculate the heat flow (q) in this case, we can use the equation:
q = m × C × ΔT
where:
q is the heat flow
m is the mass of the solution (water + NH4NO3)
C is the specific heat capacity of the solution
ΔT is the change in temperature of the solution
First, we need to find the mass of the solution by adding the mass of the water and the mass of NH4NO3:
Mass of solution = Mass of water + Mass of NH4NO3
= 42.05 g + 89.56 g
= 131.61 g
Next, we calculate the change in temperature:
ΔT = Final temperature - Initial temperature
= 7.8°C - 28.69°C
= -20.89°C
Now, we need to determine the specific heat capacity of the solution. Since the specific heat capacity of water is commonly used, we can assume it is approximately 4.18 J/g·°C.
Plugging the values into the equation, we can calculate the heat flow (q):
q = 131.61 g × 4.18 J/g·°C × (-20.89°C)
≈ -11,165 J
Therefore, the heat flow (q) in this case is approximately -11,165 Joules.
To know more about heat capacity, click here:
https://brainly.com/question/28302909
#SPJ11
What is the molecular formula of the compound with a molecular weight of 112 g/mol and percent composition: 85.6% C and 14.4% H?C8H16CH2C4H8C2H4
Answers
To find the molecular formula of this compound, what we're going to do is to follow up the steps:
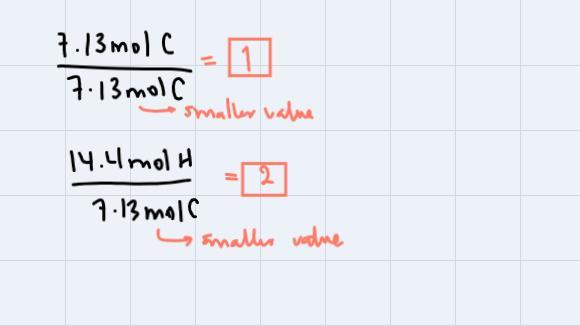
Step 1: Pass all the percentages to grams. This is, just to change the unit:
Step 2: Divide each mass by respective molar mass to obtain the moles of each element:
Step 3: Divide all the amounts in moles through by smaller value obtained:
These are the subscripts for C and H respectively. Thus our empirical formula is CH2. We're asked to find the molecular formula so, we could use the fact that the compound has a molecular weight of 112 g/mol. If we analyze, CH2 has a molecular weight of 14g/mol, so:
Step 4: We're going to divide the molecular weight of the compound with molecular formula through by the molecular weight of the compound with empirical formula:
This means that our molecular formula will be eight times the subscripts of the empirical formula. Therefore, the answer is:




a gold-colored ring has a mass of 18.9 grams and a volume of 1.12mL. Is the ring pure gold?
Answers
The gold-colored ring has a mass of 18.9 grams and a volume of 1.12mL. Is not the ring pure gold as its density is 16.87 centimeter cube.
What is density?
The density of any object is defined as the total amount of volume occupied by the mass of an object and the unit is centimeter cube.
Density = mass of substance / volume of substance
Substituting the value in formula
Density = 18.9 / 1.12mL
= 16.87 centimeter cube.
The density of pure gold must be 19 centimeter cube.
Therefore , The gold-colored ring Is not the ring pure gold as its density is 16.87 centimeter cube.
Learn more about density, here:
https://brainly.com/question/14940265
#SPJ1
10161.6 Lanthanum-140 can also emit beta radiation and change into cerium.
Complete the equation showing the decay of lanthanum (La) 140 into cerium (Ce).

Answers
Answer:
\(\frac{140}{57}La -------->\frac{140}{58} Ce +\frac{0}{-1} \beta\)
Explanation:
Beta emission occurs when a neutron is converted to a proton, an electron and a neutrino. This phenomenon reduces the neutron-proton ratio(N/P).
When a beta emission occurs, the mass number of the daughter nucleus is the same as that of the parent nucleus while the atomic number of the daughter nucleus increases by one unit.
We can see this explanation above in the reaction described in the answer section.
order the units from smallest to largest.
a. centigram
b. microgram
c. kilogram
d. milligram
e. megagram
f. nanogram
Answers
Answer:
f. nanogram
b. microgram
d. milligram
a. centigram
c. kilogram
e. megagram
Explanation:
f. nanogram = 10^-9
b. microgram = 10^-6
d. milligram = 10^-3
a. centigram = 10^-2
c. kilogram = 10^3
e. megagram = 10^6
The order of the units from smallest to largest.
f. nanogram
b. microgram
d. milligram
a. centigram
c. kilogram
What are SI units?SI units are those units that are used to measure different measurements. The SI units mean the Internation system of measurement.
A nanogram is the smallest unit of measurement. It is used to measure a very minuter amount of an object. Its value is 10⁻⁹. After this, there is a microgram, which is sued to measure micro things. Its value is 10⁻₆
Milligram, then centigram, and then there is the kilogram. The kilogram is the largest unit of measurement in the SI unit. These units are used to measure the mass of a substance.
Thus, the correct order is
f. nanogram
b. microgram
d. milligram
a. centigram
c. kilogram
To learn more about SI units, refer to the link:
https://brainly.com/question/12629581
#SPJ2
Select one option for each blank.
the brightness of a star depends on its ____(distance from earth, color, or composition), and stars that are closer look ____(brighter, dimmer, or white)
Answers
Distance from earth
Find the mass in kilograms of the liquid air that is required to produce 600L of oxygen. In normal condition, 1L of liquid air has 1.3g
Answers
Explanation:
1L is 1.3 g so 600L is 780 g
1kg= 1000g so
780 g = 0.78kg
I GUESS
Mass in kilograms of liquid air required = 0.78 kg
Given that
1 Litre of liquid air contains 1.3 grams of oxygen ( air )
Determine the amount of liquid air in Kg
volume of air given = 600 L
mass of liquid air required = x
1 litre = 1.3 grams
600 L = x
∴ x ( mass of liquid air ) = 1.3 * 600
= 780 g = 0.78 kg
Hence we can conclude that Mass in kilograms of liquid air required = 0.78 kg
Learn more about liquid air : https://brainly.com/question/636295
lead exposure can cause all of the following except ?
Answers
Lead exposure can cause all of the following except for A. Skin burns. Hence option A. is the correct answer.
What happens in lead exposure?Lead poisoning happens when lead builds up in the body over some months or years. Small amounts of lead also can cause serious health problems. Children younger than 6 years are vulnerable to lead poisoning, which affect mental and physical development.
Lead can damage the central nervous system, cardiovascular system, reproductive system, hematological system, and also kidneys. When it is absorbed into the body in high enough doses, lead can be toxic. In addition to this , workers' lead exposure can harm their children's development.
To know more about lead exposure, refer
https://brainly.com/question/15381499
#SPJ1
Note: The question given on the portal is incomplete. Here is the complete question.
Question: Lead exposure can cause all of the following except:
A. Skin burns
B. Kidney damage
C. Brain damage
D. Sterility
In an experiment, 200.00 grams of Al2O3 was decomposed producing 77.18 grams of Al. Calculate the percent yield for this experiment?
Answers
Answer:
Explanation:
Molecular weight of Al: 27
Molecular weight of O: 16
Percentage composition of Al by weight in Al2O3
= (27*2) / (27*2 + 16*3)
= 0.5294
In 200 grams of Al2O3, there are
= 200*0.5294
= 105.88 grams of Al
77.18 grams of Al was produced in the experiment.
Percent yield for the experiment
= product mass / reactant mass * 100%
= 77.18 / 105.88 * 100%
= 72.89%
How do we introduce ourselves using the periodic table?
Answers
Answer: Each element on the periodic table is listed in a box with its atomic symbol and atomic number. The element's full name and atomic mass is also sometimes indicated. The image below shows a typical entry for the element calcium. The number above the atomic symbol represents the atomic number.