____ are not used in gluconeogenesis because they are only metabolized to acetyl-CoA
Answers
Fatty acids are not used in gluconeogenesis because they are only metabolized to acetyl-CoA.
Gluconeogenesis is the process of synthesizing glucose from non-carbohydrate sources, such as amino acids, glycerol, and lactate. This process is crucial for maintaining blood glucose levels, especially during periods of fasting or low carbohydrate intake.
Fatty acids, however, cannot be directly converted into glucose because their breakdown results in the formation of acetyl-CoA. Acetyl-CoA enters the citric acid cycle (also known as the Krebs cycle or TCA cycle) and is further metabolized to generate ATP, an essential energy source for the cell. The critical step that prevents acetyl-CoA from being utilized in gluconeogenesis is the irreversible decarboxylation reaction that occurs in the citric acid cycle.
On the other hand, glycerol, a byproduct of the breakdown of triglycerides, can be used in gluconeogenesis. It is converted to dihydroxyacetone phosphate, which can then be converted into glucose. Similarly, certain amino acids can be converted into intermediates that can enter the gluconeogenesis pathway.
In summary, fatty acids are not used in gluconeogenesis because they are only metabolized to acetyl-CoA, which cannot be converted back into glucose. Instead, non-carbohydrate precursors like amino acids and glycerol are used in the gluconeogenesis process to maintain blood glucose levels.
Learn more about Gluconeogenesis here: https://brainly.com/question/29589692
#SPJ11
Related Questions
one thousand grams of water (about 1 l) contains 55.5 moles of water molecules. (one mole is 6.022 x 1023 particles). one electron is removed from every hundredth water molecule and placed in a container 100. m from the water. a. how many water molecules are there in the water container?
Answers
The number of water molecules in the container when one electron is removed from every hundredth water molecule and placed in a container is 100. m from the water is 3.30 x 1025.
Given,1 liter of water = 1000 g of water1 mole of water molecules = 6.022 x 1023 particles.
Therefore, 55.5 moles of water molecules = 55.5 x 6.022 x 1023 particles = 3.33 x 1025 particles one electron is removed from every hundredth water molecule.
So, 1 electron is removed from 1% of water molecules. Therefore, the number of water molecules from which the electron is removed = (1/100) x 3.33 x 1025 = 3.33 x 1023 molecules.
Now, this electron is placed 100 m away from the water container. The distance between the water container and the electron is not relevant for finding the number of water molecules in the container, hence can be ignored.
So, the number of water molecules in the container = Total number of water molecules - number of water molecules from which electron is removed= 3.33 x 1025 - 3.33 x 1023 = 3.30 x 1025.
Learn more about water molecules here.https://brainly.com/question/31200627
#SPJ11
An element in Group 6A (16), period 3 would have which of the following properties?
Answers
An element in group 6A, period 3 with an atomic number of 16 would be brittle in nature.
BrittlenessBrittleness is the characteristic of a substance to break easily when hit with an object or against an object.
The element with the atomic number 16 is sulfur. Sulfur does not conduct heat nor electricity and neither can it be turned into wire.
Sulfur exists naturally as a brittle solid that is yellow in appearance.
More on sulfur can be found here: https://brainly.com/question/13469437
#SPJ1
Unbend a paper clip such that
you have
a long, straight piece of metal. Bend one of the initially straight sections
back and forth several times. Does it become easier or harder to bend
the paper clip in the same spot? What happens if you keep bending the
paper clip back and forth?
uz RS nyodag
Answers
Metals have a crystalline in nature, and a consistent shift in stress causes the structure to alter, causing the metal to stiffen and fracture. This is known as metal fatige. The bending causes that to occur.
What is metal ?A metal is a substance that has a shiny look when freshly processed, polished, or shattered, and conducts electricity and heat rather effectively. Generally speaking, metals are malleable and ductile.
These characteristics are the outcome of the metallic link that exists between the metal's atoms or molecules.
The earth's crust is where most pure metals are found. They are discovered in ores, which are solid materials from which metals and minerals may be extracted. The majority of the planet's iron mass, which makes up over a third of its mass, is located in its core.
Thus, Metals have a crystalline in nature, and a consistent shift in stress causes the structure to alter.
To learn more about metal, follow the link;
https://brainly.com/question/18153051
#SPJ2
A learner was assigning oxidation numbers for different elements in the compounds OF2 and NaF. The learner assigned F an oxidation number of 1+ in OF2 and -1 in NaF.
Is this correct? Why?
A. Yes this is correct
B. No, fluorine is always assigned an oxidation number of -1.
C. No, fluorine is always assigned an oxidation number of +1.
Answers
Answer: B. No, fluorine is always assigned an oxidation number of -1.
Explanation:
4 Hydrogen has three naturally occurring isotopes. H.1, H-2, and H-3 The atomie mass of Hydrogen is 1.097 Which isotope is most abundant in nature? Explain
Answers
Answer:
The number following the name of the element is the number of subatomic particles inside the nucleus of the atom. This means that it is the mass number of the isotope. The average atomic mass of the element is the sum of the products of the percentage abundance and mass number of the naturally occurring isotopes.
Since, the average atomic mass of the hydrogen is nearest to 1 then, the most abundant isotope should be hydrogen-1.
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
Which element has a mass number of 32?
a. argon ion
b. silicon ion
C. silicon
d. sulfur
Answers
The reaction of benzaldehyde with acetone and sodium hydroxide produces ____________ This is an example of ____________ reaction.
a. stilbene
b. dibenzylideneacetone
c. benzocaine
d. anthracene
e. triphenyl methanol
Answers
The reaction of benzaldehyde with acetone and sodium hydroxide produces dibenzylideneacetone. This is an example of a condensation reaction, specifically a crossed aldol condensation.
In this reaction, benzaldehyde and acetone undergo a nucleophilic addition reaction with the strong base sodium hydroxide. The sodium hydroxide deprotonates the alpha-carbon of the carbonyl group in both benzaldehyde and acetone, creating nucleophilic enolate intermediates. The enolate of acetone then attacks the carbonyl group of benzaldehyde, forming an intermediate that subsequently loses a water molecule, leading to the formation of dibenzylideneacetone.
Dibenzylideneacetone is a yellow solid and is used as a UV absorber, as a flavoring agent, and in the preparation of fragrances. The crossed aldol condensation reaction is an important synthetic route to form α,β-unsaturated carbonyl compounds, which have numerous applications in organic chemistry.
Find out more about benzaldehyde
brainly.com/question/30725354
#SPJ4
what is Co³⁺ and Co¹⁺
Answers
Answer:
https://clever.com/
Explanation:
Why does the amplitude of a seismic wave usually decrease as the wave moves away from the epicenter?
Answers
The amplitude of a seismic wave usually decreases as the wave moves away from the epicenter due to the phenomenon of wave attenuation.
There are several factors that contribute to this attenuation:
Geometric Spreading: As the seismic wave propagates outward from the earthquake's epicenter, it spreads out over a larger area, resulting in the energy being distributed over a larger volume. This causes the amplitude of the wave to decrease with distance. According to the inverse square law, the intensity of the wave decreases with the square of the distance from the source. Absorption and Scattering: The seismic wave encounters various materials and geological structures as it travels through the Earth. These materials can absorb and scatter the energy of the wave, causing a reduction in its amplitude. Different types of rocks and soil have varying levels of attenuation, leading to differences in the decrease of wave amplitude.
Learn more about seismic wave here:
https://brainly.com/question/13056218
#SPJ11
Hey y’all is these answers correct?

Answers
Answer:
ʏᴇs, ɪ ᴛʜɪɴᴋ
Explanation:
TᕼᗩᑎKՏ ᒪOᗪՏ
source: i’m black
According to the text, which amino acid(s) contains a side chain..
a. in which molecules form covalent disulfide bridges with each other?
b. that is hydrophobic?
c. that forms hydrogen bonds?
d. that is basic?
Answers
Answer:
(b) that is hydrophobic
Explanation:
e.g, alanine
Cause, Alanine possess hydrophobic side chain and the most appropriate answer is (d) part......
Alanine is an aliphatic amino acid, because the side-chain connected to the α-carbon atom is a methyl group (-CH3), alanine is the simplest α-amino acid after glycine. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function..
Hope it will help you☺☺☺☺☺
Answer:
(b) that is hydrophobic
Explanation:
e.g, alanine
Cause, Alanine possess hydrophobic side chain and the most appropriate answer is (d) part......
Alanine is an aliphatic amino acid, because the side-chain connected to the a-carbon atom is a methyl group (-CH3), alanine is the simplest a-amino acid after glycine. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function...
Hope it helps....
Plz help asap What can you conclude about the iron(ii) and iron(iii) ions?

Answers
Answer:
The chemistry of iron is dominated by the +2 and +3 oxidation states i.e. iron(II) and iron(III) complexes e.g. Fe2+ and Fe3+ complex ions with selected ligands, usually of an octahedral shape, a few tetrahedral iron(III) complexes are mentioned too. The reactions of the aqueous ions iron(II) and iron(III) with ammonia, sodium hydroxide and sodium carbonate are described and explained as are complexes of iron(III) with the chloride ion and cyanide ion.
principal oxidation states of iron, redox reactions of iron, ligand substitution displacement reactions of iron, balanced equations of iron chemistry, formula of iron complex ions, shapes colours of iron complexes, formula of compoundsExplanation:
A strip of zinc metal was placed in a beaker that contained 120 mL of a solution of copper (II) nitrate, Cu(NO3)2(aq). The mass of the copper produced was 0.813 g. Find the initial concentration of the solution of copper (II) nitrate.
Answers
Answer:
molarity 0.1
Explanation:
mass of copper nitrate =2.4
no of moles exist in 120ml= 2.4/187.5=0.0128mole
molarity =.0.0128/.12=0.1m
Which particle model diagram represents xenon at stp?
Answers
The model that should show the corresct representation of xenon gas is one in which the gas molecules are isolated and monoatomic.
What is a noble gas?A noble gas is a member of group 18 of the periodic table. Noble gases are known not to interact with each other and occur as monoatomic particles.
The images are not shown here hence the question is incomplete. However, we do know that any of the models that show individual monoatomic particles is a representation of xenon gas.
Learn more about noble gas: https://brainly.com/question/2094768
What is the same for all of the drilling sites we examined?
Answers
Answer:
the same is what is this question like what did u exame
Which of the following is considered
individual evidence?
a. Hair with the follicle
b. Tire tracks
c. Blood type
d. Hair without the follicle
Answers
Hair with the follicle (option A) is considered individual evidence.
What is individual evidence?Individual evidence is a type of physical evidence that can be linked to a specific individual or source with a high degree of certainty. This can include items such as DNA samples, fingerprints, hair and fiber samples, shoe prints, tool marks, and other unique physical characteristics or patterns.
Individual evidence is often used in criminal investigations and trials to establish a direct connection between a suspect or perpetrator and a crime scene or victim.
Because individual evidence is typically unique to a particular person or object, it can be a powerful tool for identifying and prosecuting criminals, but it must be collected, analyzed, and presented in a careful and scientifically rigorous manner to be admissible as evidence in court.
Learn about Individual evidence here https://brainly.com/question/28888987
#SPJ1
how much PE would a 100 kg man have on a sled at the top of the hill?
Answers
The Potential energy of a 100 - kilogram man on a sled at the top of the hill would be 980H, where H is the height of the sled on the top of the hill.
What is mechanical energy?Mechanical energy is the combination of all the energy in motion represented by total kinetic energy and the total potential energy stored energy in the system which is represented by total potential energy.
As given in the problem we have to find out how much PE would a 100 kg man have on a sled at the top of the hill.
The potential energy of the man = 100 × 9.8 × H
= 980H
Both mass and acceleration due to the gravity of the earth are constant and the only variable is the height of the inclined surface.
Thus, a ball rolling down an incline has its maximum potential energy at the top, therefore the correct answer is option D.
Thus, the Potential energy of a 100 - kilogram man on a sled at the top of the hill would be 980H, where H is the height of the sled on the top of the hill.
To learn more about mechanical energy from here, refer to the link;
brainly.com/question/12319302
#SPJ2
C3.26 The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
a, Name the different elements found in this
compound.
b What is the total number of atoms present in
this molecule?
C
Between which two atoms is there a double
covalent bond?
d
How many covalent bonds does each carbon
atom make?
e
Would you expect this compound to be a solid
or a liquid at room temperature? Give a reason
for your answer.
f,
Ethanoic acid will dissolve in methylbenzene.
Would you expect the solution to conduct
electricity? Give a reason for your answer.
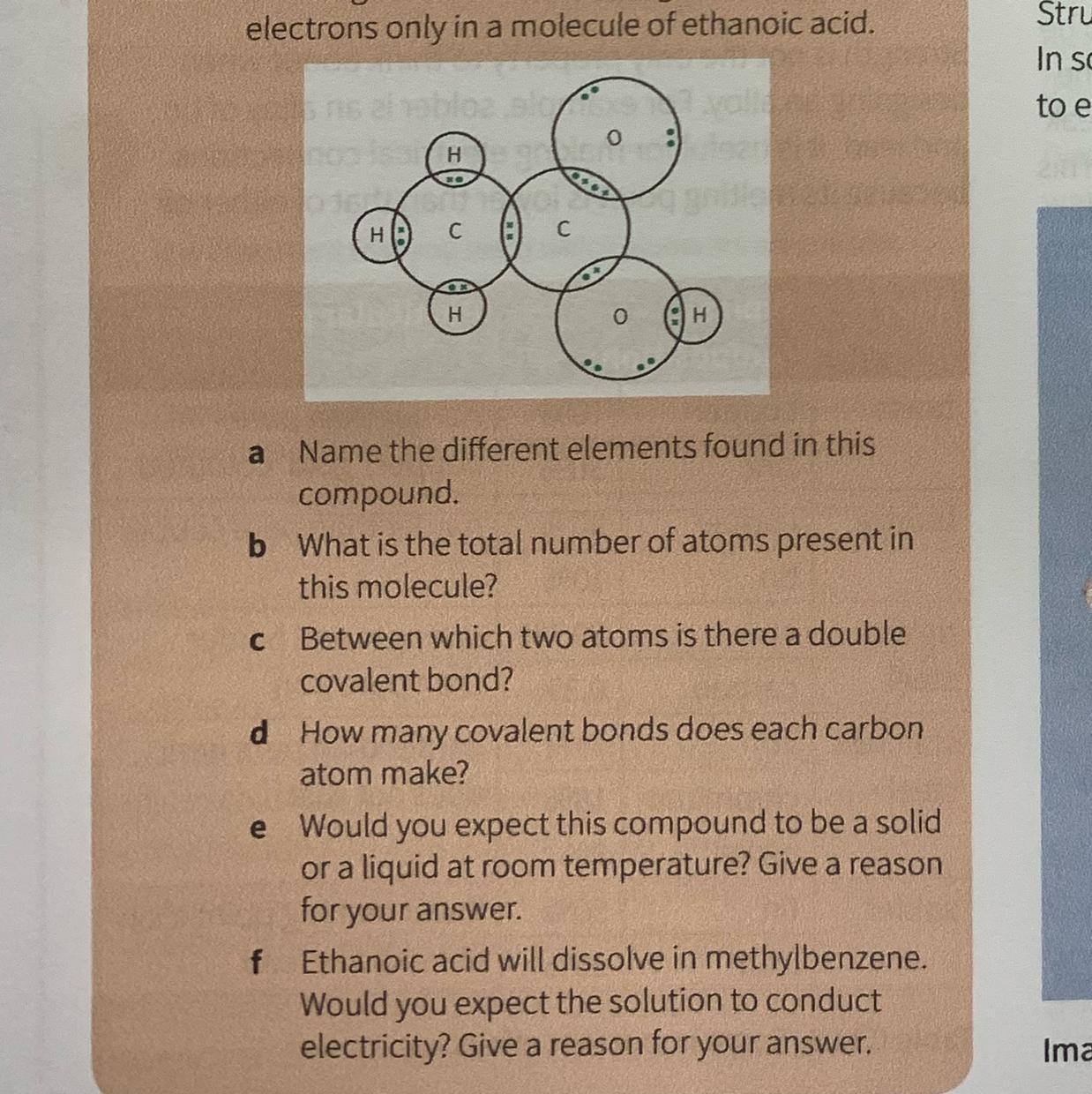
Answers
The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
elements are = Carbon, Hydrogen and oxygentotal no. of atoms = 8double bond = between one carbon and oxygentotal covalent bond = 7liquid at room temperaturea) The different elements found in this compound is Carbon, Hydrogen and oxygen.
b) The total number of atoms present in this molecule is 8.
c) Between which two atoms is there a double covalent bond is in the between of one carbon atom and oxygen atom.
d) number of the covalent bonds does each carbon atom make is 7.
e) this compound to be a liquid at room temperature
Thus, The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
elements are = Carbon, Hydrogen and oxygentotal no. of atoms = 8double bond = between one carbon and oxygentotal covalent bond = 7liquid at room temperatureTo learn more about covalent bond here
https://brainly.com/question/10777799
#SPJ1
The fact that LiBr is 7.99 % lithium and 92.01 % bromine by mass regardless of its origin is an example of _____.
Answers
LiBr being 7.99% lithium and 92.01% bromine by mass regardless of its origin is an example of a constant composition compound.
Constant composition compounds, also known as pure substances or chemical compounds, have a fixed and consistent ratio of elements by mass. This means that regardless of the source or method of production, the compound will always have the same proportion of elements. In the case of LiBr, it will always contain 7.99% lithium and 92.01% bromine by mass, regardless of where it is obtained.
This constant composition is a result of the chemical bonding between lithium and bromine atoms in LiBr. The atoms combine in a specific ratio to form a stable compound with distinct properties. The Law of Definite Proportions states that elements in a compound are always present in fixed and predictable ratios, providing a foundation for understanding constant composition compounds.
This characteristic is crucial in various fields, including chemistry, where it allows scientists to accurately predict the behavior and properties of substances. It ensures consistency in experiments, manufacturing processes, and quality control.
Learn more about Chemical compounds
brainly.com/question/33413143
#SPJ11
Is NH4NO3 → N2O 2H2O a decomposition reaction?.
Answers
Yes the above given reaction is a decomposition reaction.
A decomposition reaction can be defined as the chemical reaction in which one reactant breaks down into two or more products. The general format of a decomposition reaction is provided below.
AB -> A + B ; Where AB is the parent molecule (reactant) and A & B are the product molecules.
Disproportionation reaction, also sometimes called dismutation reaction, is basically a type of redox reaction involving simultaneous reduction and oxidation of atoms of the same element from one oxidation state (OS) to two different oxidation states.
NH4NO3 undergoes redox reaction to form N2O and Water
\(NH_{4}NO_{3}\) → \(N_{2}O\) + \(2H_{2}O\)
In this reaction, two nitrogen atoms in the reactant are present in different oxidation states. Hence, it is not a disproportionation reaction and is a decomposition reaction
To know more about decomposition reaction
https://brainly.com/question/16987748
#SPJ4
Which element below would have a 3+ charge as an ion?
Answers
Answer:
Al3+
Explanation:
Aluminium has an electronic configuration of 2, 8, 3 and the outermost valence electrons is +3 since it is a metal
Elements acquire positive or negative charges by losing or gaining electrons. The element which have 3+ charge among the options is boron (B) Hence, option B is correct.
What is boron?Boron is 5th element in periodic table and it is classified into 13th group. Boron is a metalloid and shows properties intermediate to metals and non metals.
The electronic configuration of boron is K =2, N =3. Atoms lose or gain electrons as per their valency to become stable. According to octet rule atoms become stable when their valence shell get completely filled.
Boron have 3 electrons in its valence shell. If it donate these three electrons into an electronegative atom, its K shell become its valence shell and it is completely filled. Thus boron becomes stable.]
When atoms loses electrons they acquire positive charge and this charge is numerically equal to the number of electrons lost. Thus boron acquire 3 unit of positive charge by donating its 3 electrons. Hence, option B is correct.
To find more about boron, refer the link below:
https://brainly.com/question/2790945
#SPJ6
Your question is incomplete. But your complete question probably was :
Which element below would have a 3+ charge as an ion?
answer choices.
nitrogen (N).
boron (B).
silicon (Si).
argon (Ar)
What is the molarity of a solution that contains 5.63 moles of lithium nitrate in 3.25 liters of solution?
Answers
One of the method which is used to represent the concentration of a solution is the molarity. Here the molarity of a solution that contains 5.63 moles of lithium nitrate in 3.25 liters of solution is 1.732 mol/L.
What is molarity?The ratio of number of moles of the solute present per liter of the solution is defined as the molarity. The equation which is used to calculate the molarity of a solution is:
M = n₂/ V
Here n₂ is the number of moles of the solute and 'V' is the volume of the solution in liters.
There are different methods of expressing the concentration of a solution. Molality, Normality, etc. are some examples.
The molarity of a solution that contains 5.63 moles of lithium nitrate in 3.25 liters of solution is obtained as:
M = 5.63 mol/ 3.25 L = 1.732 mol/L
Thus the molarity is 1.732 mol/L.
To know more about molarity, visit;
https://brainly.com/question/16727614
#SPJ1
How many total oxygen atoms are in 2Ba(NO3)2
Answers
Answer:
6
Explanation:
The half life of radon 222 is 3.824 days, how much time must pass of one fourth of a given amount of radon to remain?
Answers
Answer:
After 7.648 days, you will see one quarter.
Explanation:
1 → 0.5 → 0.25
Since half life of radon-222 is 3.824 days, you need twice this time to get one quarter at the end.
2 ⋅ 3.824 = 7.648 days.
four u tubes each have distilled water in the right arm, a solution in the left arm, and a semipermeable membrane between arms.
Answers
We are aware that more charged particles should be present in the most concentrated solutions (for KCl, K+, and Cl-). The increased solute concentration draws the water in via osmosis.
As a result, the water level is lowest on the right side of the U-tube (C and D). However, the left side tube (A, B) has a more concentrated solution as compared to the right side tubes (C, D).
The result is that the tube B solution is the highest concentrated.
Describe osmosis.
Osmosis is the naturally occurring net movement of solvent molecules through a selectively permeable membrane in a direction that tends to balance the solute concentrations on the two sides, from a region of high water potential (region of lower solute concentration) to a region of higher water potential.
To learn more about concentrated solution, visit:
https://brainly.com/question/10720472
#SPJ4
We are aware that more charged particles should be present in the most concentrated solutions (for KCl, K+, and Cl-). The increased solute concentration draws the water in via osmosis.
As a result, the water level is lowest on the right side of the U-tube (C and D). However, the left side tube (A, B) has a more concentrated solution as compared to the right side tubes (C, D).
The result is that the tube B solution is the highest concentrated.
Describe osmosis.
Osmosis is the naturally occurring net movement of solvent molecules through a selectively permeable membrane in a direction that tends to balance the solute concentrations on the two sides, from a region of high water potential (region of lower solute concentration) to a region of higher water potential.
Can someone explain me the energy transfer of a s’more in a campfire?
Answers
Answer:
Energy is the ability to do work, or in more simple terms: energy makes things happen. You use energy to ride your bike, play video games, bake cookies, and drive to school. Energy is exciting! Energy can be transferred from one object to another, and energy can be transferred into different forms, such as light, sound, and heat. When you sit by a campfire, you can feel the heat warm your body. The heat from the burning wood is transferred to your marshmallow, causing it to get soft and gooey. Perfect for your s’mores!
Heat can move from warm objects to cool objects, just like in the video when the heat from the wires made the paper ignite.
Explanation:
Choose the atom with:
a) Higher first ionization energy
Li and F
b) Larger atomic radius
Na ando
c) Higher electronegativity
Li and F
Answers
Answer:
a )Li
b)O
c)F
Explanation:
a) Li-1s^2 2s^1
F-1s^2 2s^2 2p^5
it is easy to pull out e- from 2p orbit than 2s because 2s orbit is close to nucleus.Therefore Li have high ionisation enthalpy
b)oxygen ion is larger than Na because o have fewer proton
c)F because it requires only 1e to achieve stable noble gas configuration.Therefore to achieve stable nobke gas electonic configuration it accept 1e.
what mass (in grams) of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution
Answers
Answer:
4.70 grams of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution.
We need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To determine the mass of NH4Cl needed to prepare the solution, we us use the formula:
m=M x V x MM ... (i)
where,
m= mass in grams
M=molarity of solution
MM= molar mass of compound
V= volume in litres
The number of moles of NH4Cl needed can be calculated using:
Moles = Molarity x Volume ...(ii)
Moles = 0.25 mol/L x 0.350 L
Moles = 0.0875 mol
Hence we can replace M x V with number of moles in equation i.
The molar mass of NH4Cl is :
Molar mass of NH4Cl = (1 x 14.01 g/mol) + (4 x 1.01 g/mol) + (1 x 35.45 g/mol)
Molar mass of NH4Cl = 53.49 g/mol
We have all the variables
Putting them in equation i.
Hence,
Mass (g) = Moles x Molar mass
Mass (g) = 0.0875 mol x 53.49 g/mol
Mass (g) = 4.68 g
Therefore, you would need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To learn more about Stoichiometry,
https://brainly.com/question/16060223
1. Which of the following species determines the chemical properties of an atom?
A. Electron
B. Neutron
C. Nucleus
D. Proton
2. The following glasswares are used to measure the volume of liquids except
A. graduated beaker
B. pipette
C. test tube
D. burette
3. Pauli exclusion principle is related to
A. quantity of electrons in the valence shell
B. filling the orbitals with lower energy first
C. the filling of degenerated orbitals
D. quantum numbers of electrons
Answers
Explanation:
The number of electrons, in turn, determines the chemical properties of the atom. Protons contribute to the mass of an atom and provide the positive charge to the nucleus. The number of protons also determines the identity of the element
Answer:
Question 1: (A)
The number of electrons determine the chemical properties of an atom.
Question 2: (C)
Test tube cannot measure the volume of liquids rather they are used in chemical reactions
Question 3: (D)
Pauli's Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers.