At high altitudes, pressure decreases to 0. 5 atm. Non-smokers can breathe 7. 2L of air per minute. How many liters of air can they breathe at sea level? (1 atm)
Answers
Non-smokers can breathe 3.6 liters of air per minute at sea level (1 atm).
At high altitudes, pressure decreases to 0.5 atm. Non-smokers can breathe 7.2L of air per minute. How many liters of air can they breathe at sea level? (1 atm)
To answer this question, we will use the Boyle's Law, which states that the product of pressure (P) and volume (V) is constant for a given amount of gas at a constant temperature. In this case, we have two different pressure conditions: high altitudes (0.5 atm) and sea level (1 atm).
We are given the volume of air breathed at high altitudes (7.2L) and asked to find the volume at sea level.
Step 1: Write down the given information:
P1 = 0.5 atm (pressure at high altitudes)
V1 = 7.2L (volume of air breathed at high altitudes)
P2 = 1 atm (pressure at sea level)
V2 = ? (volume of air breathed at sea level; this is what we need to find)
Step 2: Apply Boyle's Law:
P1 × V1 = P2 × V2
Step 3: Plug in the given values and solve for V2:
(0.5 atm) × (7.2L) = (1 atm) × V2
Step 4: Solve for V2:
V2 = (0.5 × 7.2) / 1
V2 = 3.6L
So, non-smokers can breathe 3.6 liters of air per minute at sea level (1 atm).
To know more about Non-smokers, visit:
https://brainly.com/question/13575402#
#SPJ11
Related Questions
Identify a predator from a Himalayan Mountain ecosystem. How else can this animal be classified in terms of feeding relationships? What is its prey? How else can its prey animal be classified in terms of feeding relationships?
Answers
Answer:
A snow leopard is one of the top consumers in the Himalayas who lives in dens that are close to somewhere to look down to watch its prey. They also use it's large paws to climb up slopes and snow and a long tale to balance on thin spaces to catch markhhors.
Explanation:
(:Answer:
The snow leopard is a secondary consumer in the Himalayas. They use their large paws to catch prey and have a long tail to keep their balance on balance on tight places to pounce on prey.
Explanation:
Answer my question please

Answers
Answer:
Explanation:F
a certain metal alloy is composed of 10% tin, 16% antimony, and 74% lead. if you were to have 500 g of the alloy, how many grams of tin would be found in this sample? select one: a. 10 g b. 370 g c. 80 g d. 50 g
Answers
80 grams of tin would be found in this sample.
wt% = (mass compound/mass alloy)×100
for antimony:
⇒ 16% = (g antimony / g alloy)×100
⇒ 0.16 = g antimony / g alloy
∴ g alloy = 500 g
⇒ (0.16)×(500 g) = g antimony
⇒ g antimony = 80 g
A substance that combines more than one metal or mixes a metal with other non-metallic elements is known as a metal alloy. Brass, for instance, is an alloy of the two metals copper and zinc. A metallic element (iron) and a trace amount (up to 2%) of a non-metallic element are alloyed to form steel (carbon).
An alloy's main advantages are its capacity to reduce costs while simultaneously strengthening metals while preserving their best qualities. Consider the metal iron, which has outstanding strength and durability.
To learn more about non-metallic visit:https://brainly.com/question/28650063
#SPJ4
A thermos contains 150 cm3 of coffee at 85 8C. To cool the coffee, you drop two 11-g ice cubes into the thermos. The ice cubes are initially at 0 8C and melt completely. What is the final temperature of the coffee
Answers
The final temperature of the coffee in the thermos will be a result of the heat transfer between the coffee and the melting ice cubes.
When the ice cubes are dropped into the thermos containing coffee, heat transfer occurs between the two substances until they reach thermal equilibrium. The heat transfer process involves the transfer of heat from the coffee to the ice cubes, causing them to melt.
To calculate the final temperature, we can use the principle of conservation of energy. The heat gained by the ice cubes equals the heat lost by the coffee.
First, let's calculate the heat gained by the ice cubes. The specific heat capacity of ice is approximately 2.09 J/g°C. Since each ice cube weighs 11 g and starts at 0°C, the heat gained by the ice cubes is:
Q_ice = (mass_ice) x (specific heat capacity_ice) x (change in temperature)
= (11 g) x (2.09 J/g°C) x (final temperature - 0°C)
Next, let's calculate the heat lost by the coffee. The specific heat capacity of coffee is assumed to be the same as water, which is approximately 4.18 J/g°C. The initial temperature of the coffee is 85°C, and its volume is 150 cm³. Using the density of water (1 g/cm³), we can find the mass of the coffee
mass_coffee = (density_water) x (volume_coffee)
= (1 g/cm³) x (150 cm³)
Q_coffee = (mass_coffee) x (specific heat capacity_coffee) x (final temperature - 85°C)
Since the heat gained by the ice cubes is equal to the heat lost by the coffee, we can set up the equation:
Q_ice = Q_coffee
Substituting the respective values and solving the equation will give us the final temperature of the coffee.
Learn more about:Thermos
brainly.com/question/29137878?
#SPJ11
Why can Solids Support Objects Easier Than Fluids
Answers
Answer:
because the particles in solids are more close together and strong so they would support it better than fluid because fluid particles are spread out
Explanation:
Have A Wonderful Day !!
Due to properties of solid substances, they support objects easier than liquid.
Solids can support objects easier than fluids because in solid, particles are present on fix place as compared to liquid which is not fix and can move from one place to another. In Solid substances, particles are fix so they provides better support to the objects which are placed on them.
While on the other hand, in liquid substances particles are mobile so they are not considered better supporter for objects so we can conclude that due to properties of solid substances, they support objects easier than liquid.
Learn more: https://brainly.com/question/20688004
5.23×7
estimate this product
Answers
Answer:
200
Explanation:
23 rounds down to 20
7 rounds up to 10
20 times 10 equals 200
Identify the most and the least basic compound in each of the following sets. Leave the remaining answer in each set blank. A) Sodium acetate: least v Sodium methoxide: most v Sodium phenoxide: b) Lithium ethoxide: Lithium hydroxide: Lithium formate: c) Lithium ethoxide: v Lithium diisopropylamide: Lithium acetate: Submit Answer Try Another Version 10 item attempts remaining
Answers
The most and the least basic compound in each of the following sets are as follows:
Sodium acetate: least, Sodium methoxide: mostLithium ethoxide: most, Lithium formate: leastLithium diisopropylamide: most, Lithium acetate: LeastWhen two or more elements mix chemically in a set mass ratio, the resulting product is known as a compound. Compounds are substances made up of two or more different types of elements in a predetermined ratio of their atoms. As the components join, some of their unique qualities are lost, and the newly created compound has new properties.
The property of being a base in chemistry (not an acid). A base is a material that can receive and neutralise hydrogen ions in water. The pH scale is used to quantify basicity. A pH of 7 is neutral on this scale, whereas a pH of 7 to 14 indicates increasing basicity.
Learn more about Basic compounds:
https://brainly.com/question/22713688
#SPJ4
is Dissolving of salt in water a physical or chemical change
Answers
Answer:
it is a physical change
Explanation:
When salt dissolves in water, there is a physical change. A new substance has not been created because the molecules of salt did not bond with the water
Answer:
Chemical here's why.
Explanation:
For example salt dissolving in water is usually considered to be a physical change, however the chemical species in salt solution (hydrated sodium and chlorine ions) are different from the species in solid salt.
Three safety-related rules concerning the location of machine controls on equipment involving fluid power components.
Answers
1. Ensure Clear and Visible Placement: Machine controls should be located in a position that is easily accessible, visible, and within reach of the equipment operator. Clear and intuitive labeling or color-coding can also be used to enhance visibility and assist in identifying the controls quickly.
2. Provide Adequate Guarding: The machine controls should be positioned in a manner that minimizes the risk of accidental activation or unintended operation. This can be achieved by incorporating appropriate guarding or barriers around the controls to prevent inadvertent contact or interference.
3. Consider Ergonomics and Operator Comfort: When determining the location of machine controls, it is essential to consider ergonomic principles and operator comfort. Controls should be positioned in a way that allows operators to maintain a comfortable and natural posture while operating the equipment. This can help reduce the risk of operator fatigue, musculoskeletal disorders, and errors due to discomfort or awkward reach.
These rules aim to promote operator safety, minimize the potential for accidents, and ensure efficient and effective control of equipment involving fluid power components.
To know more about musculoskeletal disorders.
https://brainly.com/question/30279097
#SPJ11
c) Ammonia is added to copper sulphate solution till excess?
Answers
Ammonia reacts with copper(II)ions to precipitate light blue copper hydroxide. Ammonia causes the copper ion to go back into the solution as a deep blue ammonia complex. The precipitate dissolves as we add an excess of ammonia.
32. List three examples of substances. Explain why each
is a substance. (3.1)
Answers
Answer: Chemical X H3 and f1
Explanation:
How many grams of sodium hydroxide are required to prepare a 200 ml solution of a 10% (weight per volume) solution? (Atomic weights: Na = 23; 0 = 16; H = 1)
Answers
Given that we want to prepare a 10% solution of sodium hydroxide in 200 mL, we need to calculate the mass of sodium hydroxide required to make this solution.
We can use the following formula to calculate the mass of solute required to make a given volume and concentration of solution:
mass of solute = volume of solution x concentration of solution x density of soluteFirst, let's calculate the density of sodium hydroxide.The density of solid NaOH is 2.13 g/mL. So, the density of sodium hydroxide solution is:
Density = 2.13 g/mLNow, let's substitute the given values into the formula to calculate the mass of sodium hydroxide required:mass of solute = 200 mL x 0.10 x 2.13 g/mL= 4.26 gTherefore, 4.26 grams of sodium hydroxide are required to prepare a 200 mL solution of a 10% (weight per volume) solution.About Sodium hydroxideSodium hydroxide, also known as lye and caustic soda or caustic soda, is an inorganic compound with the chemical formula NaOH. This compound is an ionic compound in the form of a white solid composed of the sodium cation Na⁺ and the hydroxide anion OH⁻. Sodium hydroxide is a building block that can also be found in detergents and oil stain removers. We use it to make products clean better by influencing the formula molecules, so they work better together.
Learn More About Sodium hydroxide at https://brainly.com/question/25866669
#SPJ11
A 21.3 g sample of a metal is heated to 70.0°C and dropped into 62.4 g of the water at 21.0°C. The final temperature of the water is 24.0°C. Given that the specific heat capacity of water is 4.184 Jg-1°C-1 calculate the specific heat of the metal.
Answers
heat lost by metal = heat gained by water
The heat lost by the metal can be calculated using the equation:
Q = m × c × ΔT
where:
m = mass of the metal (21.3 g)
c = specific heat of the metal (unknown)
ΔT = change in temperature of the metal (final temperature of water - initial temperature of metal)
ΔT = 24.0°C - 70.0°C = -46.0°C
Q = 21.3 g × c × (-46.0°C)
The heat gained by the water can be calculated using the equation:
Q = m × c × ΔT
where:
m = mass of the water (62.4 g)
c = specific heat of water (4.184 Jg^-1°C^-1)
ΔT = change in temperature of the water (final temperature - initial temperature)
ΔT = 24.0°C - 21.0°C = 3.0°C
Q = 62.4 g × 4.184 Jg^-1°C^-1 × 3.0°C
Since the heat lost by the metal is equal to the heat gained by the water, we can equate the two equations:
21.3 g × c × (-46.0°C) = 62.4 g × 4.184 Jg^-1°C^-1 × 3.0°C
Solving for c:
c = [62.4 g × 4.184 Jg^-1°C^-1 × 3.0°C] / [21.3 g × (-46.0°C)]
c ≈ 0.38 Jg^-1°C^-1
Therefore, the specific heat of the metal is approximately 0.38 Jg^-1°C^-1.
1. The author says that bog bodies were discovered as long ago as the 1600s, but the only ones existing today are those found after the late 1800s. What hap- pened to the earlier bog bodies?
Answers
Answer:
The earlier bog bodies that were discovered in the 1600s might have not been preserved properly due to a lack of knowledge on how to preserve them or a lack of awareness of their significance. It is also possible that they might have decayed and decomposed over time and not survived till the present day. However, the bog bodies found after the late 1800s were preserved and studied extensively due to the increasing awareness and understanding of their historical and archaeological significance.
Explanation:
Hope this helped!! Have a great day/night!!
What is the reason behind that the melting and boiling point of iron is more than that of sodium? Write any two differences between metalloids and alloys.
Answers
Answer:
difference between metal and alloy is that the metal is a pure substance whereas the alloy is a mixture of two or more components.
Explanation:
Mostly the metalloids have the appearance just like the metallic appearance And also they are the brittle one's . Boron and silicon are the example. Note; By combining with other metals metalloid can form the alloy
the density of a liquid is 1.09 g/ml. what is the mass of a 27.3 ml sample of this liquid in units of g?
Answers
The density of a liquid is 1.09 g/ml. what is the mass of a 27.3 ml sample of this liquid in units of g is 2.507 g
we know that, ρ = \(\frac{M}{V}\)
Here,
ρ = density of the substance
M = mass of the substance
V = Volume of the substance
given , density ρ = 1.09 g/ml
volume V =27.3 ml
Then, the mass of the liquid can be given by,
M = ρ × V
M = 1.09 g/ml × 27.3 ml
M = 2.507 g
Thus ,the mass of the liquid in g is 2.507 g
Learn more about density of substances,
https://brainly.com/question/31521608?referrer
A car tire has a volume of 32.2 L with a pressure of 34.5 psi when the temperature is 27°C. If the temperature increases to 43° and the volume decreases to 31.04, What is the new pressure?
Answers
Answer:
Using the combined gas law:
(P1 x V1) / T1 = (P2 x V2) / T2
where:
P1 = 34.5 psi (initial pressure)
V1 = 32.2 L (initial volume)
T1 = 27°C + 273.15 = 300.15 K (initial temperature in Kelvin)
V2 = 31.04 L (final volume)
T2 = 43°C + 273.15 = 316.15 K (final temperature in Kelvin)
Solving for P2:
(P1 x V1 x T2) / (V2 x T1) = P2
(34.5 psi x 32.2 L x 316.15 K) / (31.04 L x 300.15 K) = P2
P2 = 37.2 psi
Therefore, the new pressure in the tire is 37.2 psi when the temperature increases to 43°C and the volume decreases to 31.04 L.
Plz answer quick 15 points!!! Will give brainiest!!!
Hailey and Bailey compare two samples of matter. They make a table on paper to record the properties they can observe or see of each sample. Which property provides the best evidence that both samples are liquids and NOT solids?
A) mass
B) shape
C) color
D) volume

Answers
Answer:
B) Shape
Explanation:
The property of shape provides the best evidence that both samples are liquids and not solids because liquids do not have a definite shape, in contrast to solids, which do.
the least reactive in oxygen in the is
Answers
Answer:
Now we can say the silver, gold and platinum do not react with oxygen because these three compounds are least reactive and present in downwards in the reactivity series.
Explanation:
if it helped you please mark me a brainliest :))
what carbonyl compound and alcohol are formed by hydrolysis of each acetal
Answers
Acetals can be hydrolyzed using catalytic acid to produce a carbonyl compound and alcohol. If the acid concentration is increased, acetal can be hydrolyzed back to its initial aldehyde or ketone form.
This mechanism occurs in the opposite direction of the acetal formation mechanism. The hydrolysis of each acetal generates a carbonyl compound and an alcohol.What are Acetals?Acetals are organic compounds that are formed by the reaction of an aldehyde or ketone with two molecules of alcohol, and they have the following general structure: R1R2C(OR')2.Acetals can be regarded as derived from hemiacetals, which are formed by the reaction of an aldehyde or ketone with one molecule of alcohol.The carbonyl carbon in an acetal is bonded to two alkoxide (OR) groups, while the carbonyl carbon in a hemiacetal is bonded to only one. As a result, acetals are more stable than hemiacetals. Acetals are widely used in organic synthesis, including as protecting groups for carbonyl groups in reactions that would otherwise destroy them.Example:Acetal hydrolysis occurs when an acid catalyst is used to cleave the two ether bonds in the molecule. When an acetal is hydrolyzed with an acid catalyst such as H2SO4, a carbonyl compound and an alcohol are formed.Example:H2SO4 is added to the acetal, which hydrolyzes it, producing an aldehyde or ketone and two alcohol molecules. For example, if dimethyl acetal is hydrolyzed, it will yield acetone and two methanol molecules.
To know more about molecules , visit ;
https://brainly.com/question/475709
#SPJ11
2KCIO3 -> 2KCI+ 302
How many moles of oxygen are produced by
the decomposition of 6.0 moles of potassium
chlorate, KCIO3?
Answers
Answer:
9 moles
Explanation:
The balanced chemical equation for this decomposition reaction is as follows:
2KCIO3 → 2KCI+ 302
Based on this equation, 2 moles of potassium chlorate (KCIO3) decomposes to form 3 moles of oxygen gas (O2).
Hence, 6 moles of pottasium chlorate will decompose to produce;
6 × 3 ÷ 2
= 18 ÷ 2
= 9 moles of O2.
Could someone help me with this pls i need it asap chimestry
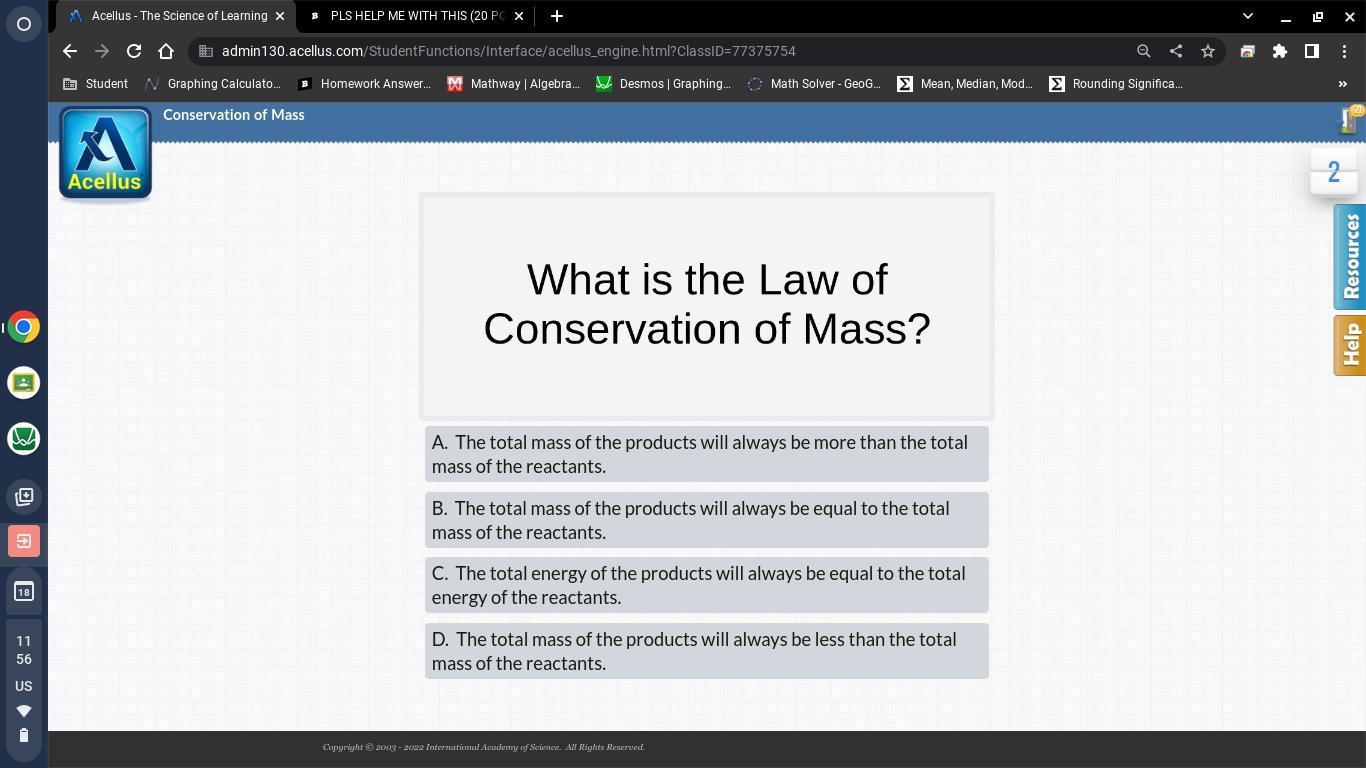
Answers
Answer:
b
Explanation:
the mass stays the same, it is only rearanged
Answer: B
Explanation: Law of Conservation of Mass states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
What is the carbon dioxide lewis structure?
Answers

Why does the flow rate of a fluid with large particles increase?
NEED ANSWER ASAP!!!
Answers
Answer:
The viscosity of intestinal contents should define the mixing, diffusion and flow of nutrients and other materials in digesta (1–4). The viscosity of intestinal contents has been measured only after the removal of solid particles by centrifugation (5,6), under the assumption that particles or insoluble fibers in the diet do not affect the viscosity of gut contents (7). However, the complete removal of solid particles considerably reduced the coefficient of viscosity of pig cecal contents and changed the contents from a non-Newtonian to a Newtonian fluid in our recent study
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
What theoretical yield of NaCl would result from reacting 3.00 moles of Na2CO3 with excess HCl (aqueous)?
Answers
Answer:
350.7 g of NaCl
Explanation:
For this reactions our reactants are:
HCl and Na₂CO₃.
Then, the reaction is:
2HCl + Na₂CO₃ → 2NaCl + H₂CO₃
2 moles of hydrochloric acid react to 1 mol of sodium carbonate in order to produce 2 moles of salt and 1 mol of carbonic acid.
Ratio is 1:2. 1 mol of sodium carbonate can produce 2 moles of NaCl
Then, 3 moles of carbonate may produce (3 . 2) /1 = 6
We convert the moles to mass to determine the theoretical yield
6 mol . 58.45g /mol = 350.7 g
The theoretical yield of NaCl produced is 350.7 g.
What is NaCl?NaCl is a salt, and it is an ionic compound.
The reaction is
\(\rm 2HCl + Na_2CO_3 \rightarrow 2NaCl + H_2CO_3\)
2 mol of HCl reacts with 1 mol of \(Na_2CO_3\) and produces 2 mol of NaCl and 1 mol of \(H_2CO_3\).
The ratio will be \(Na_2CO_3\) : NaCl
1 : 2
Then, for 3 mol of \(Na_2CO_3\)
\(\dfrac{3 \times 2}{1} = 6\)
Now, convert the moles to mass
\(6\; mol \times 58.45g /mol = 350.7 g\)
Thus, the theoretical yield produced is 350.7 g.
Learn more about NaCl
https://brainly.com/question/1473063
About how old is the Sun?
1.2 billion years
4.6 billion years
6.4 billion years
8.2 billion years
Answers
Answer:
B : 4.6 billion years
Credits go to the person above me.
;)
Explanation:
EDGE 2021
The sun is about 4.6 billion years old. Therefore, option (B) is correct.
What is the sun made of?The Sun is made primarily of the elements hydrogen and helium. They account for 74.9% and 23.8% mass of the Sun in the photosphere. All heavier elements, called metals account for less than 2% of the mass, with oxygen (<1%), carbon (0.3%), neon (0.2%), and iron (0.2%) being the most abundant.
The hydrogen and helium in the Sun would have been produced by Big Bang nucleosynthesis and the heavier elements were produced by former generations of stars before the Sun was formed.
The amount of helium and its location within the Sun has gradually changed over the past 4.6 billion years. The proportion of helium has raised from about 24% to 60% due to fusion, and some of the He and heavy elements have settled from the photosphere toward the center due to gravity.
Therefore, the age of the sun is about 4.6 billion years.
Learn more about Sun, here:
https://brainly.com/question/2526507
#SPJ6
3. A student obtains the number 0.045006700 on a calculator. If this number actually has four significant figures, how should it be
written?
A. 0.4567
B. 0.4501
C. 0.045
D. 0.04500
E. 0.04501
Answers
Explanation:
leading zeros do not count as significant figures, Hence, this number correct to four significant figures should be written as in option E.
Cifras significativas de 63,000
Answers
Answer:
Cifras significativas de 63,000
Result 63000
Sig Higos 2 (63000)
Decimales 0
Notación cientifica 6.3 × 104
Notación electrónica 6.3e+4
Palabras sesenta y tres mil
What are the characteristic property's of metals such as malleability and high conductivity are due to ?
Answers
Answer:
Metallic Bonding
Explanation:
Conductivity occurs when electrons or ions are free moving. The conductivity in metals is due to electrons being delocalized in a sea of electrons. Metals contain metallic bonding which allows the electrons to freely move.
Malleability happens when adjacent layers of positive ions move relative to one another while being in contact with the sea of electrons from the metallic bonding.

