At which point does a planet move most slowly in its orbit , at aphelion or perihelion
Answers
At aphelion, when the planet is farthest from the Sun, its velocity is the slowest in its orbit. Conversely, at perihelion, the point in the orbit where the planet is closest to the Sun, the planet moves fastest.
A planet moves most slowly in its orbit at aphelion. Aphelion refers to the point in a planet's orbit where it is farthest from the Sun.
As a planet orbits the Sun, it experiences gravitational attraction, causing it to accelerate as it gets closer to the Sun and decelerate as it moves away.
Aphelion refers to the point in an object's orbit around the Sun where it is farthest from the Sun. It is the point in an object's elliptical orbit where the distance between the object and the Sun is at its maximum.
To learn more about aphelion, follow the link:
https://brainly.com/question/30583998
#SPJ12
Related Questions
How many molecules of H₂O are
equivalent to 97.2 g H₂O?
(H = 1.008 g/mol, O = 16.00 g/mol)
[?]
[?]x10 molecules
Enter your answer with the correct number of
significant figures and be sure the prefix is a number
between 1 and 10!
dro's Number = 6.02 x 1023 molecules/mole
Answers
Answer:
3.25 x 10²⁴ molecules H₂O
Explanation:
To find the number of H₂O molecules, you need to (1) convert grams to moles (via molar mass) and then (2) convert moles to molecules (via Avogadro's Number). It is important to arrange the ratios/conversions in a way that allows for the cancellation of units. The final answer should have 3 sig figs to match the amount of sig figs in the given value.
Atomic Mass (H): 1.008 g/mol
Atomic Mass (O): 16.00 g/mol
Molar Mass (H₂O): 2(1.008 g/mol) + 16.00 g/mol
Molar Mass (H₂O): 18.016 g/mol
Avogadro's Number:
6.02 x 10²³ molecules = 1 mole
97.2 g H₂O 1 mole 6.02 x 10²³ molecules
------------------ x ------------------- x ------------------------------------ =
18.016 g 1 mole
= 3.25 x 10²⁴ molecules H₂O
Earth is removed from the top to reveal the coal. Which type of coal mine does this describe? undersea subterranean drill strip
Answers
Answer:
Strip
Explanation:
Strip mining removes Earth in long strips from the top to reveal the coal. This earth to be removed is known as 'overburden'. The overburden from the first strip is referred to as out-of-pit dumping. Strip mining is a part of surface mining.
Strip mining destroys forests, landscapes, forests and wildlife habitats as trees and plants are removed from the mining area.
Therefore,
this describes the Strip coal mine.
Answer:
strip Mining.
Explanation:
help meeeeeeeee pleaseeeeeeeeeee

Answers
What unit is used to measure weight?
Answers
The unit of measurement for weight is that of force, which in the International System of Units (SI) is the newton. For example, an object with a mass of one kilogram has a weight of about 9.8 newtons on the surface of the Earth, and about one-sixth as much on the Moon.
Answer:
The unit is: Newton.
Hope it helps:D
y'all need to help me please

Answers
Explanation:
2. The periodic table of elements is a tabular representation of chemical elements, organized on the basis of their atomic numbers
3. a. Mg
c.Fe
b.K
d. Cu
4. a.carbon
b.chlorine
c.aluminum
d.strontium
5. a. 5 group 18 element
b. 5 group 13 element
d. 5 group 17 element
c. 5 group 1 element
6. a. 3 group
b. 4 group
c. 5 group
d.4 group
7. I don't know
and.... you're welcome :))❤️


Guys help me pls
Which of type of elements are mainly gases at room temperature, dull and
brittle with low melting points?

Answers
according to the information in the passage, in general, adding electrons to nonmetals is:
Answers
According to the information, in general, adding electrons to nonmetals is called reduction.
Nonmetals are elements in the periodic table that are generally not very reactive chemically.
In general, nonmetals have a low melting and boiling point, are poor conductors of heat and electricity, and have a tendency to gain electrons when they react with other elements.
Nonmetals typically form negative ions (anions) when they react with metals, which means they gain electrons.
This is called reduction, which occurs when electrons are added to an atom, reducing its oxidation state or oxidation number.
Additionally, nonmetals often form covalent bonds with other nonmetals by sharing electrons to form molecules.
This is in contrast to metals, which typically form ionic bonds by transferring electrons to form cations and anions.
To know more about electrons, visit:
https://brainly.com/question/12001116
#SPJ11
Help needed ASAP, I will mark your answer as brainliest.

Answers
Answer:
D
Explanation:
^
Answer:
the answer is D
Explanation:
I hope this helped.
What is the [OH) if the poH is 4.9?
a) 4.9 x 10-10 M
Ob) 1.0 x 10-4 M
C) 1.25 x 10-5 M
O d) 7.94 x 10-10 M
Answers
Answer:
the answer is 4.9×10-10M
Which applies to fusion? Check all that apply.
involves the splitting of nuclei
takes place in the Sun
releases radiation as a waste product
occurs in nuclear power plants and is used to generate electricity
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
Answers
Fusion Nuclear fusion is a response wherein two or greater atomic nuclei are blended to shape one or greater different atomic nuclei and subatomic debris. It
1. involves the splitting of nuclei
2. releases large amounts of energy
4. releases radiation as a waste product
5. occurs in nuclear power plants and is used to generate electricity.
Fusion reactions power the sun and other stars. In a fusion reaction, light nuclei merge to form a single heavier nucleus. The technique releases electricity due to the fact the total mass of the resulting unmarried nucleus is much less than the mass of the two unique nuclei. The leftover mass becomes energy. The difference in mass between the reactants and merchandise is manifested as either the discharge or absorption of power.
Learn more about Fusion here:-https://brainly.com/question/17870368
#SPJ9
Answer:
2, 5, 6
Explanation:
edge 2023
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
What is the independent variable in this experiment?
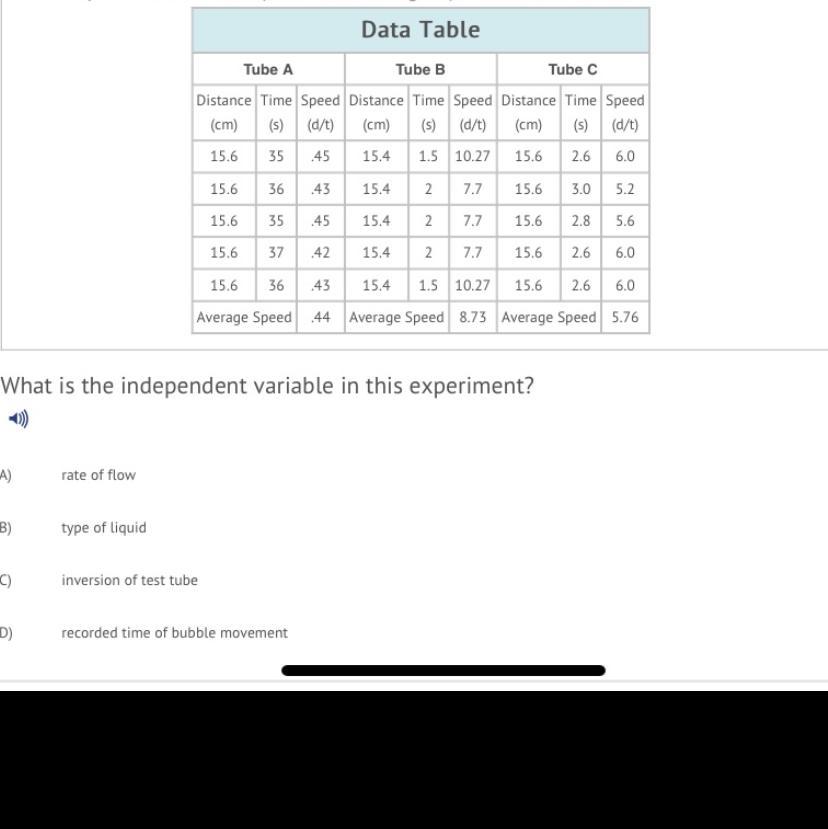
Answers
Answer:
D
Explanation:
because it was the variable that was changed in the experiment. distance was controlled and speed changed with time.
Identify which of the following functions are eigenfunctions of the operator d/dx: (a) eᶦᵏˣ, (b) cos kx, (c) k, (d) kx, (e) e⁻ᵃˣ^². Give the corresponding eigenvalue where appropriate.
Answers
Among the given functions, (a) e^ikx, (c) k, and (d) kx are eigenfunctions of the operator d/dx. The corresponding eigenvalues are (a) ik, (c) 0, and (d) k, respectively.
To determine whether a function is an eigenfunction of the operator d/dx, we need to check if applying d/dx to the function yields a scalar multiple of the original function.
(a) For the function e^ikx, we have d/dx(e^ikx) = ike^ikx. This shows that e^ikx is an eigenfunction of d/dx with an eigenvalue of ik.
(b) Taking the derivative of cos(kx) with respect to x gives us -ksin(kx), which is not proportional to the original function. Therefore, cos(kx) is not an eigenfunction of d/dx.
(c) For the constant function k, d/dx(k) = 0. This implies that k is an eigenfunction of d/dx with an eigenvalue of 0.
(d) The function kx has a derivative of d/dx(kx) = k. Hence, kx is an eigenfunction of d/dx with an eigenvalue of k.
(e) The derivative of e^(-ax^2) with respect to x is -2axe^(-ax^2). Since -2ax is not proportional to e^(-ax^2), e^(-ax^2) is not an eigenfunction of d/dx.
(a) e^ikx, (c) k, and (d) kx are eigenfunctions of d/dx, with corresponding eigenvalues of (a) ik, (c) 0, and (d) k, respectively. On the other hand, (b) cos(kx) and (e) e^(-ax^2) are not eigenfunctions of d/dx.
To learn more about eigenfunctions here brainly.com/question/2289152
#SPJ11
Write the entire chemical equation and label the reactants and products.
C3H8 + 5 O2 → 3 CO2 + 4 H2O
Answers
Answer:the equation is already balanced and the reactants are the C3H8+ 5 O2 and the products are 3CO2+ 4H2O
Explanation:
"dairy cattle management question
Typical DMI is ________% during the dry period
Answers
Typical DMI (Dry Matter Intake) for dairy cattle during the dry period is around 2.0 to 2.2% of their body weight. This means that a dairy cow will consume approximately 2.0 to 2.2% of its body weight in dry feed each day during the dry period.
Dairy cows are not producing milk during the dry period, which is the time frame of about 60 days prior to calving, and are instead getting ready for the upcoming lactation cycle. Their dietary needs at this period are different from those they have when they are actively making milk.
There are a number of reasons why DMI is lower during the dry season. First of all, during the dry phase, the cow's udder involutes (regresses) and gets ready for the impending lactation. The cow's metabolic activity decreases as a result, and she requires less energy.
Second, during the dry season, the cow's rumen capacity drops. Due to the growing calf and the shrinking amount of the udder, the capacity of the rumen, the main component of the cow's stomach, has been diminished. The amount of feed that the cow can eat is constrained by the small rumen space.
The cow's physiological state during the dry period and fluctuations in hormone levels can also have an impact on the cow's appetite. A decrease in feed intake may result from hormonal changes and rumen motility changes that the cow may encounter.
It is crucial to keep in mind that the precise DMI during the dry period can change depending on elements including cow genetics, physical condition, and management techniques. For dairy farmers to maintain ideal cow health and get their animals ready for a successful lactation phase, it's critical to track each cow's consumption and modify feeding techniques accordingly.
To know more about cows:
https://brainly.com/question/33570860
#SPJ4
Question 32
The regulatory level for benzene under the RCRA Toxicity Characteristic rule is
a. 0.50 mg/l
b. 100 mg/kg
c. 0.2 mg/l
d. 25.0 mg/l
Answers
The regulatory level for benzene under the Resource Conservation and Recovery Act (RCRA) Toxicity Characteristic rule is 0.5 mg/L.
The RCRA is a United States federal law that governs the management of hazardous waste, and the Toxicity Characteristic rule is a provision of the RCRA that sets limits on the concentration of certain hazardous constituents in waste.
Benzene is a known human carcinogen and is commonly found in industrial waste. The Toxicity Characteristic rule regulates the concentration of benzene and other hazardous constituents in waste to minimize their impact on human health and the environment.
The regulatory level of 0.5 mg/L means that if the concentration of benzene in a waste sample exceeds this level, the waste is considered hazardous and must be managed accordingly. The RCRA also sets requirements for the treatment, storage, and disposal of hazardous waste to prevent contamination of the environment and protect public health.
To learn more about benzene
https://brainly.com/question/13731563
#SPJ11
How many grams are there in 8.25 L of oxygen gas (O2)
hey guys show work I don't know what's going on... also does the number 22.4 have to be in there somehow???? send help
Answers
There are approximately 11.78 grams of oxygen gas (O2) in 8.25 L at STP.
What is the mass of 8.25L of oxygen at STP?
To calculate the number of grams of oxygen gas (O2) in 8.25 L, we need to use the ideal gas law which states:
PV = nRT
Where;
P is the pressure of the gas, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.Assuming standard temperature and pressure (STP) conditions (0°C and 1 atm), we can use the molar volume of a gas at STP, which is 22.4 L/mol, to calculate the number of moles of oxygen gas in 8.25 L:
n = (V / V_m) = (8.25 L) / (22.4 L/mol) = 0.3683 mol
The molar mass of O2 is approximately 32 g/mol, so we can calculate the number of grams of oxygen gas in 0.3683 mol:
mass = n x molar mass = 0.3683 mol x 32 g/mol = 11.78 g
Learn more about mass of oxygen here: https://brainly.com/question/12902286
#SPJ1
What is the concentration of a 53.05 mL solution of HBr that is completely titrated by 33.50 mL of a 0.200 M NaOH solution
Answers
The concentration of the HBr solution is 0.112 M.
To determine the concentration of the HBr solution, we can use the concept of stoichiometry and the balanced chemical equation for the reaction between HBr and NaOH:
HBr + NaOH -> NaBr + H2O
From the balanced equation, we can see that the ratio of moles of HBr to moles of NaOH is 1:1. Therefore, the number of moles of HBr in the titrated solution is equal to the number of moles of NaOH used.
First, we calculate the number of moles of NaOH used:
moles of NaOH = volume of NaOH solution (L) x concentration of NaOH (M)
= 0.03350 L x 0.200 M
= 0.00670 moles
Since the ratio of moles of HBr to moles of NaOH is 1:1, the number of moles of HBr is also 0.00670 moles.
Next, we calculate the concentration of the HBr solution:
concentration of HBr = moles of HBr / volume of HBr solution (L)
= 0.00670 moles / 0.05305 L
= 0.126 M
Therefore, the concentration of the HBr solution is 0.126 M, or 0.112 M when rounded to three significant figures.
To learn more about concentration, here
https://brainly.com/question/3045247
#SPJ4
ultiple qualitative tests can be used to determine the properties of carbohydrate samples. identify the test that provides the given information about carbohydrates. identify reducing sugars choose... distinguish between monosaccharides and disaccharides choose... distinguish between a pentose and a hexose choose... determine whether starch is present
Answers
1. To identify reducing sugars, use the Benedict's test.
2. To distinguish between monosaccharides and disaccharides, use the Barfoed's test.
3. To distinguish between a pentose and a hexose, use the Seliwanoff's test.
4. To determine whether starch is present, use the Iodine test.
1. Benedict's test: This test detects the presence of reducing sugars, which have free aldehyde or ketone groups. When heated with Benedict's reagent, reducing sugars react and produce a color change ranging from green to red-orange, depending on the sugar concentration.
2. Barfoed's test: This test differentiates monosaccharides from disaccharides. When heated with Barfoed's reagent, monosaccharides react quickly and form a red precipitate, while disaccharides react more slowly or not at all.
3. Seliwanoff's test: This test is used to distinguish between pentoses and hexoses. When heated with Seliwanoff's reagent, pentoses produce a red color, while hexoses produce a yellow color.
4. Iodine test: This test detects the presence of starch. When iodine solution is added to a sample containing starch, the solution turns a blue-black color.
By using the Benedict's, Barfoed's, Seliwanoff's, and Iodine tests, you can identify reducing sugars, distinguish between monosaccharides and disaccharides, differentiate between pentoses and hexoses, and determine the presence of starch in carbohydrate samples.
For more information on test for carbohydrates kindly visit to
https://brainly.com/question/29655942
#SPJ11
Using the balanced equation
2 C,H, (9) + 5 0, (g) - > 4 CO, (g) + 2
H,O(g)
How many moles of water (H,O) are
produced when 25.0 grams of CH, are
consumed?
Answers
When 25.0 grammes of Methane are consumed, 2.3385 moles of water are created.
Does one mole weigh one gramme?The ratio between the atomic mass unit and gramme mass unit sizes affects the number in a mole, or Avogadro's number. One mole of hydrogen atoms weighs around one gramme, compared to the mass of one hydrogen atom, which is roughly one unit.
2 CH3CH3(g) + 5 O₂(g) → 4 CO₂(g) + 6 H₂O(g)
The molar mass of Methane is 16.04 g/mol, so 25.0 grams of CH₄ is equal to:
25.0 g / 16.04 g/mol = 1.559 mol CH₄
From the balanced equation, the molar ratio of CH₄ to Water is 2:3. Therefore, for every 2 moles of Methane consumed, 3 moles of Water are produced.
So, for 1.559 mol of CH₄ consumed, the amount of Water produced would be:
3/2 x 1.559 mol = 2.3385 mol
To know more about Methane visit:-
https://brainly.com/question/28933327
#SPJ1
movement of ions produces epsps in cochlea hair cells
Answers
The movement of ions that produces EPSPs (Excitatory Postsynaptic Potentials) in cochlear hair cells is called mechanotransduction.
EPSPs (Excitatory Postsynaptic Potentials) are produced in cochlea hair cells due to the movement of ions. Cochlea hair cells are responsible for converting mechanical sound waves into electrical signals that can be interpreted by the brain. When sound waves reach the hair cells, they cause the movement of the fluid in the inner ear, which then causes the hair cells to bend.
The bending of the hair cells opens ion channels, which allows positive ions like potassium and calcium to flow into the hair cells, producing an electrical signal. This electrical signal triggers the release of neurotransmitters, which then stimulate the nearby auditory nerve fibers to transmit the signal to the brain. The movement of ions and the resulting electrical signals are essential for hearing and for the perception of sound.
To learn more about mechanotransduction, here
https://brainly.com/question/13022742
#SPJ4
A backdraft explosion can occur when
- There is a lack of fuel in a partially-burned room
- There is a lack of oxygen in a partially-burned room
- A door is opened into a room instead of opening into a hallway or outside
- A fire is very smoky
Answers
A backdraft explosion can occur when there is a lack of oxygen in a partially-burned room. This situation can be exacerbated if a door is opened into the room, introducing a sudden supply of oxygen, which then leads to a rapid combustion of the remaining fuel, causing the explosion. A fire being very smoky may also indicate a lack of oxygen, increasing the risk of a backdraft explosion.
A backdraft explosion can occur under certain conditions such as when there is a lack of fuel in a partially-burned room or a lack of oxygen in a partially-burned room. Additionally, if a door is opened into a room instead of opening into a hallway or outside, it can create a draft that can lead to a backdraft explosion. Furthermore, a fire that is very smoky can also lead to a backdraft explosion as the smoke can build up and ignite when oxygen is suddenly introduced. It is important to be aware of these potential dangers and to take necessary precautions to prevent a backdraft explosion from occurring.
Learn more about backdraft explosion at brainly.com/question/28256373
#SPJ11
For the equation SnO2 + 2H2 Sn + 2H2O, tin (IV) oxide reacts with excess hydrogen to produce tin and water. What is the limiting reactant?
A) SnO2
B) H2
C) Sn
D) H2O
Answers
Answer:
SnO2
Explanation:
The limiting reactant is the reactant that runs out first during a chemical reaction.
Reactants are on the left side of the reaction, so SnO2 and H2. They tell us in the question that there is "excess" hydrogen (H2), so we know there is plenty of it, as "excess" means "more than enough." If we have more than enough hydrogen, then we know that we will run out of SnO2 first.
:)
Answer:SnO2
Explanation:
the belt of maximum solar energy input to earth swings back and forth ________.
Answers
The belt of maximum solar energy input to earth swings back and forth through the tropics.
Solar energy is the energy derived from the sun, or rather, from the heat produced by sun. This heat/radiation is propagated in the form of electromagnetic radiations. The amount of solar radiation that reaches any given area on the Earth's surface is not constant - it fluctuates. Although, every location receives some sunshine during the course of a year. This radiation is captured using solar technology, which transforms it into useful energy for different purposes. Photovoltaics (PV) and concentrating solar-thermal power (CSP) are the two main solar technologies used. If we observe the movement of earth around the sun, we know that the tropics (around the equator) receive maximum sunlight throughout the year, wheres, the poles receive the minimum. Hence, The belt of maximum solar energy input to earth swings back and forth through the tropics.
To know more about solar energy, click here : https://brainly.com/question/27344926
#SPJ4
At what temperature will uranium hexafluoride , the densest gas known have the same average speed as a molecule of the lightest gas, hydrogen at 37 degree celcius
Answers
Answer:
the required temperature of uranium hexafluoride is 54156.25 K
Explanation:
Given the data in the question;
We know that average speed is;
u = \([ 3RT / MM ]^{1/2\) ----------- let this be equation 1
where MM is the molar mass
T is temperature
R is universal gas constant and u is the average speed.
First we get the average speed of H₂
\(U_{H}\)₂ = \([ 3RT_H_2 / MM_H_2 ]^{1/2\) ------ let this be equation 2
Next is the average speed of UF₆
\(U_{UF\)₆ = \([ 3RT_{UF_6} / MM_{UF_6} ]^{1/2\) ------ let this be equation 3
given that; both have the same average speed, equation 2 = equation 3;
\([ 3RT_H_2 / MM_H_2 ]^{1/2\) = \([ 3RT_{UF_6} / MM_{UF_6} ]^{1/2\)
we multiply both sides by 1/3R and also square both sides.
\([ T_H_2 / MM_H_2 ]\) = \([ T_{UF_6} / MM_{UF_6} ]\)
given that; temperature of hydrogen T\(_{H\)₂ = 37°C = ( 37 + 273.15)K = 310.15 K
we know that Molar mass of H₂; MM\(_{H\)₂ = 2.016 g/mol
and molar mass of UF₆; MM\(_{UF\)₆ = 352.02 g/mol
so we substitute
[ 310.15 K / 2.016 g/mol ] = [ T\(_{UF\)₆ / 352.02 g/mol ]
T\(_{UF\)₆ = [ 352.02 g/mol × 310.15 K ] / 2.016 g/mol
T\(_{UF\)₆ = 109179.003 K/ 2.016
T\(_{UF\)₆ = 54156.25 K
Therefore, the required temperature of uranium hexafluoride is 54156.25 K
2. Scientists learn this from fossils EXCEPT:
O How old the organism could be
O the climate of the area from the time
O What future fossils will look like
O the structure of the creature
Answers
All fossils can teach scientists something, but What fossils of the future will look like. They can discover information on the structure of the organism, its age potential, and the local climate.
What distinguishes an organic structure from an inorganic one?The main distinction between these inorganic compounds molecules is that organic molecules always contain an atom of carbon, whereas the majority of inorganic compounds do not. A simple C-H bond or carbon-hydrogen are present in almost all organic molecules.
What elements make up an organic structure?The primary ingredients of the majority of organic compounds are four elements: hydrogen, carbon, oxygen, and nitrogen.
To know more about organic structure visit:
https://brainly.com/question/14364464
#SPJ1
An example of a heterogeneous mixture is
A)
soil
B)
sugar
C)
carbon dioxide
D)
carbon monoxide
Answers
Air can also be an example of a heterogeneous mixture
Atomic radii decrease, moving from left to right across a period. As a result, the electrons become closer to the nucleus. What effect does this movement have on the ionization energy (the amount of energy required to remove an electron from an atom)? The ionization energy stays the same. The ionization energy decreases. The ionization energy increases. Electrons have no effect on ionization energy.
Answers
Answer:
The ionisation energy increases.
Explanation:
This is because the force of attraction between the electrons and the positive nucleus will increase
Balance the equation: FeSO4(s) + O2(g) + H2O(l) = Fe(OH)3 + H2SO4
Answers
Answer: 4 FeSO4(s) + O2(g) + 10 H2O(l) ---> 4 Fe(OH)3 + 4 H2SO4
Explanation:
FeSO4(s) + O2(g) + H2O(l) = Fe(OH)3 + H2SO4
In other to balance the equation, the total number of atoms of each element on the Left Hand Side (LHS) of the equation must be equal to the total number of atoms on the Right Hand Side (RHS) of the equation.
Add the following coefficients to the equation;
FeSO4 = Add 4, H20 = Add 10, Fe (OH)3 = Add 4 and H2SO4 = Add 4
By doing this, you will discover that the total number of atoms on bothe sides of the equation are balanced; thus we have;
4 FeSO4(s) + O2(g) + 10 H2O(l) ---> 4 Fe(OH)3 + 4 H2SO4
In which one of the following branches of natural science are properties of materials studied?
a.
Biology
b.
Chemistry
c.
Geology
d.
Astronomy
Answers
Answer:
chemistry
Explanation:
it is the study of structer, composition and change that matter undergoes