If a gas occupies 30 L at STP, what would be the volume if the temperature was raised to 323. 15K ?
Answers
At STP, typically defined as a temperature of 0°C (273.15K) and a pressure of 1 atm, the volume of a gas is equal to 30 L.
When the temperature of the gas is increased, the kinetic energy of the gas particles increases, causing them to move more quickly and expand. This expansion of the gas increases its volume.
Using the ideal gas law, the new volume of the gas can be calculated by multiplying the original volume by the ratio of the new temperature (323.15K) to the original temperature (273.15K) and raising that to the power of 1/273.15.
In this case, the new volume of the gas is 33.53 L. In conclusion, when the temperature of a gas is raised, its volume increases.
Know more about Ideal gas law here
https://brainly.com/question/28257995#
#SPJ11
Related Questions
According to the dramatic exposition at the beginning of the play, what fueled the Salem witch hunt depicted in the play?
Answers
People who had "wronged" them want revenge and their own brand of justice.
What do you call anyone who is dramatic?Melodramatic is often compared to theatrical, histrionic, and theatrical. Melodramatic conjures up excessive emotionalism or improper theatricalism, even if all these adjectives refer to "has a tone or an impact like that of staged plays."
What causes somebody to be so dramatic?Acting excessively theatrical is a symptom of hysterical personality disorder (HPD). People who have HPD may well have superficial, erratic moods and rely on their outside looks to attract attention. They could also be sensitive to other influences and struggle to develop their own personalities.
To know more about dramatic visit:
https://brainly.com/question/29236164
#SPJ4
What is the percent water in the compound CaCl2 2H2O ?
Answers
Answer:
147.1g
Explanation:
Which statement defines specific heat capacity for given sample?
O the temperature of a given sample
1 %
the temperature that a given sample can withstand
O the quantity of heat that is required to raise the sample's temperature by 1°C (Kelvin)
O the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure
Answers
The correct statement about specific heat capacity is " the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure".
What is specific heat capacity ?The heat capacity of a given sample of a substance divided mostly by the mass of the sample, also known as mass heat capacity, is the specific heat capacity of that substance.
What is heat ?Heat is the energy transmitted from one body to the other as a result of a temperature differential. When two bodies of different temperatures collide, energy is transmitted.
Therefore, the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure is correct statement.
To know more about heat and specific heat capacity.
https://brainly.com/question/13860901
#SPJ2
Answer: D. the quantity of heat that is required to 1 g of the sample 1°C (Kelvin) at a constant pressure
Explanation:
Specific heat capacity is defined as the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (Kelvin) at a constant pressure.
It is dependent on temperature, especially for gases, and is related to intermolecular forces. The higher the specific heat of a substance, the more energy is needed to raise the temperature of the substance.
You can multiply this value by the mass of a given substance (g) and the change in temperature (degC) to find the amount of heat gained or lost (Joules).




at the end of the experiment you titrate the solution with 0.507 m hcl and it takes 38.30 ml to neutralize the ammonia. what is the equilibrium molarity of ammonia after the 2nd addition of ammonia? (report your answer with 4 decimal places.)
Answers
The equilibrium molarity of ammonia after the 2nd addition of ammonia is 1.94181× 10⁻⁶ M.
The total number of moles of solute in a particular solution's molarity is expressed as moles of solute per litre of solution. As opposed to mass, which fluctuates with changes in the system's physical circumstances, the volume of a solution depends on changes in the system's physical conditions, such as pressure and temperature. M, sometimes known as a molar, stands for molarity. When one gramme of solute dissolves in one litre of solution, the solution has a molarity of one.
The balanced equation of reaction is given below;
HCl + NH₃ → NH₄Cl.
We are given the volume in milliliters, let us convert them into Litres;
= 38.30 × 10⁻³ Litres.
Here, we have an incomplete question (one parameter is missing- the volume of ammonia,NH₃). Therefore, we assume that the volume of Ammonia, NH₃ is 10mL(10× 10⁻³ Litres).
Step one: we need to calculate the number of moles of HCl.
Number of moles of HCl= molarity × volume.
Number of moles of HCl= 0.507 M × 38.30× 10⁻³ L.
Number of moles of HCl= 0.0194181 moles.
From the equation of reaction above, we have that one mole of ammonia is reacting with one mole of Hydrogen Chloride, HCl. Hence, the number of moles of ammonia is equal to the number of moles of Hydrogen Chloride, HCl.
Step two: calculate the molarity of Ammonia, NH₃.
The molarity of ammonia= number of moles of ammonia/ volume of Ammonia, NH₃.
Molarity of Ammonia= 0.0194181/10× 10⁻³ moles NH₃.
Molarity of Ammonia= 0.00000194181.
Molarity of Ammonia = 1.94181× 10⁻⁶ M.
Learn more about Molarity:
https://brainly.com/question/26528084
#SPJ4
If the reaction has reached equilibrium and then the sealed reaction vessel expands, increasing the volume, the reaction would ____.
Answers
If the reaction has reached equilibrium and then the sealed reaction vessel expands, increasing the volume, the reaction would increase.
The equilibrium will change to favor the direction that produces more moles of gas when the volume is increased.
Equilibrium is a state of balance between opposing forces or actions that can be static (such as when forces are applied to a body and the resultant is zero) or dynamic (such as during a reversible chemical reaction when the velocities in both directions are equal). It can also be a state of equilibrium between the mind and the body.
In terms of economics, equilibrium occurs when supply and demand are equal. When you are composed and steady, you are in an equilibrium state.
To learn more about equilibrium please visit -
https://brainly.com/question/13458865
#SPJ4
Which qualities of an effective digital leader are being demonstrated by Caleb? Check all that apply. Problem-solving skills good time-management overseeing group tasks effective scheduling excitement about the project.
Answers
The effective leader qualities demonstrated by Caleb are problem-solving skills, good time management, and effective scheduling. Thus, options A, B, and D are correct.
The project led by Caleb is based on natural resources. The team plays an important task in completing important and achieving the targets and goals for a project plays.
What are the qualities of Caleb?Caleb's team has lost focus on the project. The team leader is responsible to manage his team and encouraging them to achieve targets.
Caleb manages his team by arranging meetings and analyzing members' progress.
The leadership qualities that are demonstrated by Caleb are problem-solving skills, good time management, and effective scheduling. Thus, options A, B, and D are correct.
Learn more about leadership qualities, here:
https://brainly.com/question/2779795
Answer:
a & e
Explanation:
I really need help with the science work

Answers
Answer:
A. in the cells of autotrophs
Explanation:
Photosynthesis takes place in the cells of autotrophs. Autotrophs are capable of manufacturing their own food by two major means which is by photosynthesis or by chemosynthesis.
In photosynthesis green plants manufacture their foods using sunlight to synthesize energy rich compounds from carbon dioxide and water. The product is often times glucose and oxygen gas.Heterotrophs on the other hand depends on the food manufactured by the autotrophs for their nourishment
The ozone hole is largely a consequence of the use of __________.
a. nitrogen oxides
b. fossil fuels
c. chlorofluorocarbon refrigerants
d. carbon monoxide and carbon dioxide
Answers
The ozone hole is largely a consequence of the use of chlorofluorocarbon refrigerants. Therefore, option C is correct.
What is ozone layer?The ozone layer can e described as a region of Earth's stratosphere that absorbs the Sun's ultraviolet radiation. Ozone layer contains a high concentration of ozone to other parts of the atmosphere, although small in relation to other gases in the stratosphere.
The ozone layer consists less than 10 ppm of ozone, while the average ozone concentration in atmosphere as a whole is about 0.3 ppm. The ozone layer is found in the lower portion of the stratosphere, from approximately 15 to 35 km above Earth, although its thickness varies seasonally as well as geographically.
The ozone layer can be depleted by man made compounds, such as chlorofluorocarbons (CFCs) and bromofluorocarbons.
Learn more about ozone layer, here:
https://brainly.com/question/14330630
#SPJ1
which phrase describes carbon-14 dating? uses the rate at which nitrogen-14 decays into carbon-14 dates the remains of organisms to determine when they died measures the number of unstable elements in ancient volcanic rocks
Answers
The phrase that describes carbon-14 dating is "decays into carbon-14 dates the remains of organisms to determine when they died" (Option B).
Carbon-14 dating is a method used to determine the age of organic materials by measuring the amount of carbon-14 in the sample and comparing it to the initial amount of carbon-14 when the organism was alive. As carbon-14 decays over time, the ratio of carbon-14 to carbon-12 in the sample changes, allowing scientists to calculate how long ago the organism died. It is not a method used to measure the number of unstable elements in ancient volcanic rocks.
Your options are incomplete, but most probably your options were
A) uses the rate at which nitrogen-14
B) decays into carbon-14 dates the remains of organisms to determine when they died
C) measures the number of unstable elements in ancient volcanic rocks
D) determines the age of ancient fossils older than 50,000 years old
Thus, the correct option is B.
Learn more about Carbon-14 dating: https://brainly.com/question/17830972
#SPJ11
Answer: B.
Explanation:

Consider the reaction. KCI + Pb(NO3)2 — KNO3 + PbCI2, Identify which species will precipitate in aqueous solution. KNO3 PbCl2 Pb(NO3)2 КСІ
Answers
The species that will precipitate in aqueous solution when KCI and Pb(NO3)₂ react is PbCl₂.
When KCI and Pb(NO3)₂ react, they form KNO₃ and PbCl₂. PbCl₂ has a solubility of 4.6 g/L, while KNO₃ has a solubility of 311.7 g/L.
means that when the two species are placed in aqueous solution, the PbCl₂ will not be able to dissolve, and will instead form a precipitate.
This is because the solubility of PbCl₂ is much lower than that of KNO₃. As such, the only species that will precipitate in aqueous solution is PbCl₂.
To know more about precipitate click on below link:
https://brainly.com/question/30904755#
#SPJ11
If solutions of NH4OJ and CaS are mixed, what is the name and formula of the precipitate formed?

Answers
When NH₄OH and CaS are mixed the following reaction occurs:
NH₄OH + CaS ---> Ca(OH)₂ + (NH₄)₂S
Ca(OH)₂ is a poorly soluble base in water. So the formula of the precipitade formed is Ca(OH)₂ and its name is calcium hydroxide.
Bases with cations of the group II of the periodic table are generally poorly soluble in water.
im struggling and dont want to fail

Answers
Answer: Hey I am sure you will do great just try your best
Explanation:
Q4 This question relates the combustion reactions of acetylene, hydrogen and ethane. (a) Express the stoichiometric ecpigtions for the combustion reactions of acetylene, hydrogen and ethane with their respective standard heats of combustion obtained from physical property table. (b) Verify the standard heat of combustion of acetylene in Q4(a) by using heat of formation method. (c) The equation below shows the acerylene hydrogenation reaction: C2H2(g)+2H2(g)→C2H6(g) (i) Compute the standard heat of acetylcne hydrogenation reaction using tabulated heats of formation and heats of combustion. (ii) Verify the answer in Q4(e)(1) by using Hess's Law.
Answers
Stoichiometric equations for the combustion reactions ΔHf° (C2H2) = (2 x (-393.5)) + (-285.8) - (-1299.5) = +226.7 kJ mol-1(c) Acetylene hydrogenation reaction
Acetylene combustion reaction:C2H2 (g) + (5/2) O2 (g) → 2 CO2 (g) + H2O (l) ΔHc° = -1299.5 kJ mol-1 Hydrogen combustion reaction:2H2 (g) + O2 (g) → 2 H2O (l) ΔHc° = - 483.7 kJ mol-1Ethane combustion reaction:C2H6 (g) + (7/2) O2 (g) → 2 CO2 (g) + 3 H2O (l) ΔHc° = - 1560 kJ mol-1(b) Heat of formation method for verifying the standard heat of combustion of acetylene: The standard heat of combustion of acetylene from the heat of formation method is:ΔHc° (C2H2) = 2 ΔHf° (CO2) + ΔHf° (H2O) - 2 ΔHf° (C2H2) = -1299.5 kJ mol-1ΔHf° (CO2) = -393.5 kJ mol-1ΔHf° (H2O) = -285.8 kJ mol-1.
For verifying the answer in Q4(e)(1) using Hess's Law, we need to convert acetylene hydrogenation reaction into a combination of other reactions:Reaction 1:C2H2 (g) + (2.5) O2 (g) → 2 CO2 (g) + H2O (l) ΔH1 = -1299.5 kJ mol-1Reaction 2:2 CO2 (g) + 2.5 H2 (g) → C2H6 (g) + 5 O2 (g) ΔH2 = +1560 kJ mol-1After multiplying and adding the above equations, we get the required reaction as:C2H2 (g) + 2 H2 (g) → C2H6 (g) ΔH = -396.1 kJ mol-1.
To know more about reactions visit:
https://brainly.com/question/16737295
#SPJ11
Determine the number of protons, neutrons, and electrons in the following: 65 29X
Answers
The number of protons is 29, the number of neutrons is 36 and the number of electrons is 29 in \(X_{65}^{29}\).
To determine the number of protons, neutrons, and electrons in an atom, you need the atomic number (Z) and the mass number (A) of the element.
In the case of \(X_{65}^{29}\), the atomic number (Z) is 29, which corresponds to the number of protons in the nucleus of the atom. The total of protons and neutrons is represented by the mass number (A), which is 65.
Number of protons = Atomic number (Z) = 29
Number of neutrons
\(= Mass number (A) - Number of protons\)
= 65 - 29 = 36
When an atom is neutral, the number of electrons and protons in the atom are equal. So, in this case, there are 29 electrons.
Therefore, there are 29 protons, 36 neutrons, and 29 electrons in a nucleus.
Learn more about atomic number here:
https://brainly.com/question/14719614
#SPJ11
Suppose you want to search for high-redshift star-forming galaxies using a telescope equipped with a spectrograph able to measure the entire optical spectrum (400-700 nm). Star-forming galaxies contain copious hydrogen gas, from which stars form. Some of this gas will be ionized by the newly-formed stars; the spectra of star-forming galaxies there exhibit bright hydrogen lines in emission. Light from the newly-formed stars is absorbed by neutral hydrogen gas as it passes through the galaxy. a) If you wish to search for Lyman-a emitting galaxies, over what redshift range can you find such galaxies? [3 points] b) If you detect only one line, you cannot be certain that this line is the Lyman-a line, and hence that the galaxy is indeed at the computed redshift. Assuming you can also observe in the infrared (wavelengths >700 nm), how can you change your strategy to make sure that the line you detect is really the Lyman-a line? Give three examples of how you can increase confidence in the correct identification of the Lyman-a line considering only hydrogen gas. [3 points] c) Limited only to optical wavelengths and considering only hydrogen gas, what strategy should you adopt to be certain that the line you detect is really the Lyman-a line while maximizing the redshift range over which you find galaxies? With this strategy, over what redshift range can you find star-forming galaxies? Justify through appropriate computations and reasoning that this is in fact the optimal strategy for maximizing the redshift range of your search. [10 points] d) Apart form hydrogen emission lines, star-forming galaxies also usually exhibit bright [OIII] forbidden lines. Why can such lines be seen from interstellar gas but not the Earth's atmosphere or in the laboratory? [4 points]
Answers
a) The maximum observable range of redshifts that produces Lyman-alpha line is 0 ≤ z ≤ 10.6
b) i) identifying the galaxy with a radio source, ii) looking for other Lyman lines, iii) a coincidence with a continuum break
c)The maximum redshift range over which galaxies can be found using this strategy is z = 7 to z = 15.5.
d)Earth's atmosphere absorbs the radiation, and the laboratory conditions are not the same as interstellar conditions.
a) Lyman-alpha line is produced by the hydrogen atoms that have electrons that are in the ground state being raised to the first excited state. Over a certain range of redshifts, the Lyman-alpha line is redshifted to longer wavelengths that are observable by an optical spectrograph. The maximum observable range of redshifts that produces Lyman-alpha line is 0 ≤ z ≤ 10.6 (depending on the exact details of the galaxy's emission profile).
b) Observing the galaxy in the infrared can help in the identification of the Lyman-alpha line as it is shifted to longer wavelengths. Three ways to increase confidence in the correct identification of the Lyman-alpha line are:
i) identifying the galaxy with a radio source, ii) looking for other Lyman lines, iii) a coincidence with a continuum break.
c) The strategy that needs to be adopted is to look for the Lyman limit, which is the point at which the spectrum is cut off by the absorption of all hydrogen in the galaxy. To be certain that the line you detect is the Lyman-alpha line, you need to look for a decrement in the flux of the galaxy at wavelengths shorter than the line and a decrement in the flux at wavelengths longer than the line. This is because the Lyman limit will be shifted to longer wavelengths at higher redshifts, so to maximize the redshift range over which galaxies can be found, you need to search for the Lyman limit at the longest wavelength possible. The maximum redshift range over which galaxies can be found using this strategy is z = 7 to z = 15.5.
d) The reason why such lines can be seen from interstellar gas but not the Earth's atmosphere or in the laboratory is that the Earth's atmosphere absorbs the radiation, and the laboratory conditions are not the same as interstellar conditions. The forbidden lines from the interstellar gas are not affected by dust absorption because they are produced in regions where dust is not present.
for such more questions on atmosphere
https://brainly.com/question/19587559
1.How far can you travel if you drive 4 hours and can average 120 Km/hr?
a.30 Km
b.480 Km
c.80 Km
Answers
Answer:
B
Explanation:
D=S×T
=120km/h×4hrs
=480km
Answer:
the answer is b
Explanation:
this is the answer :)
Could someone help me answer these questions with the answer and typed steps for how each answer was found? I asked this question previously but, I could not read the handwritten answer.
7. A 25 g soil sample was extracted with 75 mL of NH4OAc (pH 7.0), and the filtrate was analyzed
on an atomic absorption unit. The following results were obtained:
100 mg/L Ca2+, 45 mg/L Mg2+, 85.5 mg/L K+, 94.2 mg/L Al3+ and 8.0 mg/L H+.
a. What is the CEC in cmol(+)/kg for this sample?
b. What is the % B.S. for this soil?
c. What is the % acid saturation for this soil sample?
Answers
The CEC for this soil sample is 675.2 cmol(+)/kg.
The % Base Saturation for this soil sample is approximately 136.62%.
The % Acid Saturation for this soil sample is approximately 60.55%.
To calculate the CEC, % Base Saturation (B.S.), and % Acid Saturation for the given soil sample:
a. Calculation of CEC (Cation Exchange Capacity):
CEC is the sum of exchangeable cations in the soil. From the given results, we have:
CEC = Ca2+ + Mg2+ + K+ + Al3+
CEC = (100 mg/L + 45 mg/L + 85.5 mg/L + 94.2 mg/L) / (25 g / 1000)
CEC = 168.7 mg / (25 g / 1000)
CEC = 675.2 cmol(+)/kg
b. Calculation of % Base Saturation (B.S.):
% B.S. represents the percentage of CEC occupied by base cations. In this case, we consider Ca2+, Mg2+, and K+ as base cations. The formula to calculate % B.S. is:
% B.S. = (Ca2+ + Mg2+ + K+) / CEC * 100
% B.S. = (100 mg/L + 45 mg/L + 85.5 mg/L) / (168.7 cmol(+)/kg) * 100
% B.S. = 230.5 mg / (168.7 cmol(+)/kg) * 100
% B.S. = 136.62%
c. Calculation of % Acid Saturation:
% Acid Saturation represents the percentage of CEC occupied by acid cations, in this case, H+ and Al3+. The formula to calculate % Acid Saturation is:
% Acid Saturation = (H+ + Al3+) / CEC * 100
% Acid Saturation = (8.0 mg/L + 94.2 mg/L) / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 102.2 mg / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 60.55%
Please note that the given values were in milligrams per liter (mg/L), and the CEC and % Saturation values were calculated assuming a conversion from mg/L to cmol(+)/kg using the mass of the soil sample (25 g).
TO know more about CEC (Cation Exchange Capacity)
https://brainly.com/question/30689981
#SPJ11
B-
Why many nuclei like U234, U236 and
U238 undergo fission only by fast neutrons?
Answers
The nucleus more easily, increasing the probability of causing fission many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission can be caused by slow as well as fast neutrons. It is the energy of the neutron which determines its effectiveness in causing fission. Fast neutrons are more effective in causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission is a nuclear reaction process in which the nucleus of an atom is split into two smaller nuclei with the release of a large amount of energy and two or three neutrons. Uranium-235 (U-235) and Plutonium-239 (Pu-239) are the most commonly used fissile materials, but other materials like U-234, U-236, and U-238 can also undergo fission. When a neutron is absorbed by the nucleus of a fissile material like U-235 or Pu-239, it becomes unstable and splits into two smaller nuclei with the release of a large amount of energy.
The fission process also releases two or three neutrons, which can cause further fission of other nuclei, leading to a chain reaction. The chain reaction can be controlled by using a neutron moderator, which slows down the fast neutrons, making them more effective in causing fission. The efficiency of the fission reaction depends on the energy of the neutron.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy. This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy.
This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Learn more about nucleus with the given link,
https://brainly.com/question/13061744
#SPJ11
What is a theory?
Group of answer choices
another word for hypothesis
an educated guess
a possible explanation of a general principle offered to explain observed facts
a possible explanation for a hypothesis

Answers
An explanation for a natural occurrence is provided by a hypothesis, which is supported by observation and testing.
A hypothesis is a well-informed prediction based on specific data that serves as the basis for more research.
Many of them dismiss evolution as "simply a hypothesis," in contrast to gravity, which must exist because it is a law. In the scientific community, the terms "theory," "facts," "laws," and "hypothesis" have extremely particular meanings that don't quite correspond to the ones we use in ordinary language. A testable hypothesis is a speculative explanation for an observable. It serves as a springboard for additional explanation. Contrarily, a theory is an explanation of a particular feature of the natural world that is supported by facts, validated hypotheses, and rules.
Learn more about hypothesis here:
https://brainly.com/question/17173491
#SPJ9
which molecule listed below has a nonpolar covalent bond? group of answer choices none of the options h2o all of the options nh3 h2
Answers
H2 is the molecule between the possibilities of having a non-polar covalent bond.
Two identical hydrogen atoms with the same electronegativity form the compound H2. In a covalent bond the electron pair is shared equally because there is little difference in electronegativity of the two hydrogen atoms, leading to a non-polar covalent bond.
Because of the difference in electronegativity between the hydrogen and oxygen/nitrogen atoms, H2O and NH3 have polar covalent bonds. A polar covalent bond is formed when the electron pair in the covalent bond is shared unequally by the oxygen and nitrogen atoms, which are more electronegative than hydrogen.
So, the correct option is C.
Learn more about covalent bond, here:
https://brainly.com/question/19382448
#SPJ4
Your question is incomplete, most probably the complete question is:
Which molecule listed below has a nonpolar covalent bond?
group of answer choices
h2o nh3 h2all of the options none of the optionsReaction between silver nitrate and sodium chloride. A balanced chemical equation
Answers
Answer:
NaCl(aq) + AgNO3(aq) Sodium Silver chloride nitrate AgCl(s) + NaNO3(aq) Silver Sodium chloride nitrate
the phase diagram for sulfur is shown below. the rhombic and monoclinic phases are two solid phases with different structures. which of the phases of sulfur is most dense?
Answers
Therefore, monoclinic sulfur is the denser phase.
The phase diagram for sulfur is shown below. The rhombic and monoclinic phases are two solid phases with different structures. Among the phases of sulfur, the most dense phase is Monoclinic sulfur phase.
A phase diagram is a graphical representation of the physical state of matter with temperature and pressure as variables, in this case, the physical state of sulfur.
The phase diagram of sulfur is shown below:
The diagram indicates that sulfur can exist in various states, including solid, liquid, and gaseous states. The rhombic and monoclinic phases are two solid phases with different structures. Among the two phases, the monoclinic sulfur phase is the most dense phase.
Let us explore both of the phases and their densities to better understand the situation:
Rhombic sulfur has a density of 2.08 g/cm³.
Monoclinic sulfur has a density of 2.29 g/cm³.
Therefore, monoclinic sulfur is the denser phase.
To know more about monoclinic visit:
https://brainly.com/question/29750483
#SPJ11
When small molecules are linked together to form larger molecules, the increase in entropy typically comes from:
a. light
b. enzymes
c. work
d. gas
e. heat
Answers
When small molecules are linked together to form larger molecules, the process typically releases heat and increases the entropy of the system.
The option e. heat is correct.
The increase in entropy results from the release of heat energy as a result of the formation of new bonds between the atoms in the small molecules. This process is known as exothermic, which means that heat is released to the surrounding environment.
It's important to note that the relationship between entropy and heat is based on the Second Law of Thermodynamics, which states that the total entropy of a closed system will always increase over time.
To learn more about entropy, visit:
https://brainly.com/question/22145368#
#SPJ11
what is amajor disturbance that caused the ecosystem to stabilize at a new equilibrium.
Answers
Fires, landslides, flooding, windstorms, and insect and pest outbreaks were among the major ecological disturbances that caused the ecosystem to stabilize at a new equilibrium.
What does it mean when an ecosystem reaches a new equilibrium?An ecosystem is said to have ecological stability (or equilibrium) if it can return to its equilibrium state after a perturbation (a capacity known as resilience) or if it does not experience unexpected large changes in its characteristics over time.Natural ecosystems are frequently extremely sensitive to change, such as the introduction or extinction of a species. A healthy ecosystem is said to be in equilibrium, a relatively stable state in which population sizes remain within a sustainable range (not too many of a certain species alive or dead).Ecosystems must have the right balance of non-living elements such as sunlight and water. To maintain equilibrium, they must have the proper balance of different species.To learn more about equilibrium refer to :
https://brainly.com/question/1083607
#SPJ1
One difference between mixtures and pure substances is that
A)
mixtures can be physically separated.
B)
mixtures are made of one type of atom.
pure substances have no chemical bonds
D)
pure substances can be physically separated,
ning
Answers
Answer:
I think b but I could be wrong
Answer:
a
Explanation:
i just took it
Make a circle graph for each set of data. Top 5 Most Linked-To Sites on the World Wide Web Site Hits (millions) America Online (www.aol.com) 93.0 Microsoft (www.msn.com) 83.8 Yahoo! (www.yahoo.com) 80.2 Terra Lycos (www.lycos.com) 40.3 About (www.about.com) 36.6 Source: Scholastic Kid’s Almanac, 2001 a. A circle graph. America Online is about 25 percent; Microsoft, 25 percent; Yahoo 23 percent; Terra Lycos, 15 percent; About, 10 percent. c. A circle graph. America Online is about 27 percent; Microsoft, 27 percent; Yahoo 22 percent; Terra Lycos, 12 percent; About, 10 percent. b. A circle graph. America Online is about 12 percent; Microsoft, 10 percent; Yahoo 27 percent; Terra Lycos, 17 percent; About, 23 percent. d. A circle graph. America Online is about 12 percent; Microsoft, 10 percent; Yahoo 25 percent; Terra Lycos, 25 percent; About, 20 percent. Please select the best answer from the choices provided A B C D
Answers
Answer:
A
Explanation:
(Q2) A liquid mixture containing 40 mol% benzene and 60 mol% toluene is fed to a distillation column. The overhead product is nearly pure benzene and the bottoms product, pure toluene. The reboiler is heated by steam condensing at 140 ∘
C at the rate of 80 kg for each kilogram mole of feed. The overhead condenser is cooled by water at the essentially constant temperature of 20 ∘
C. Neglecting heat losses and sensible heat effects and assuming that the fed mixture is an ideal solution, calculate the total change in entropy resulting from separation of one kmole of feed.
Answers
The total change in entropy resulting from the separation of one kmole of feed is about -0.079 kJ/K.
Given information: The liquid mixture containing 40 mol% benzene and 60 mol% toluene is fed to a distillation column. The overhead product is nearly pure benzene and the bottoms product, pure toluene.
The reboiler is heated by steam condensing at 140 ∘C at the rate of 80 kg for each kilogram mole of feed. The overhead condenser is cooled by water at the essentially constant temperature of 20 ∘C.
Neglecting heat losses and sensible heat effects and assuming that the fed mixture is an ideal solution, calculate the total change in entropy resulting from the separation of one kmole of feed.
Entropy is a measure of the energy in a system that cannot be used to do work.
The change in entropy is given by ΔS = Q/T, where ΔS is the change in entropy, Q is the heat transferred, and T is the temperature of the system.
The heat transferred can be found using the formula Q = H1 - H2, where H1 is the enthalpy of the feed and H2 is the enthalpy of the products.
The change in entropy resulting from the separation of one kmole of feed is calculated as follows:
Calculate the enthalpy of the feed. The enthalpy of a mixture of two components can be calculated using the formula H = x1*H1 + x2*H2, where x1 and x2 are the mole fractions of the components, and H1 and H2 are the enthalpies of the pure components. The enthalpy of the feed is given by:
Hf = xbenzene*Hbenzene + xtoulene*Htoulene
where xbenzene = 0.4 and xtoulene = 0.6 are the mole fractions of benzene and toluene, respectively.
Hbenzene = 30.85 kJ/mol and Htoulene = 36.56 kJ/mol are the enthalpies of pure benzene and toluene, respectively.
Hf = 0.4*30.85 + 0.6*36.56 = 34.82 kJ/mol.
Calculate the enthalpy of the products. The enthalpy of the overhead product (benzene) is given by:
Hov = Hbenzene = 30.85 kJ/mol
The enthalpy of the bottoms product (toluene) is given by:
Hbt = Htoulene = 36.56 kJ/mol
Calculate the heat transferred.
The heat transferred is given by Q = Hf - Hov - Hbt
= 34.82 - 30.85 - 36.56
= -32.59 kJ/mol.
Calculate the temperature of the system.
The temperature of the system is given by the temperature of the steam condensing in the reboiler, which is 140 ∘C.
Convert this to Kelvin by adding 273.15 K: T = 140 + 273.15 = 413.15 K.
Calculate the total change in entropy. The total change in entropy is given by ΔS = Q/T = -32.59/413.15 = -0.079 kJ/K.
The total change in entropy resulting from the separation of one kmole of feed is about -0.079 kJ/K.
To know more about entropy, visit:
https://brainly.com/question/20166134
#SPJ11
The diagram shows the electron configuration of an atom of an element for the electrons in the s and p orbitals.
What is the group number of the element in the periodic table?
A) 1
B) 2
C) 13
D) 15
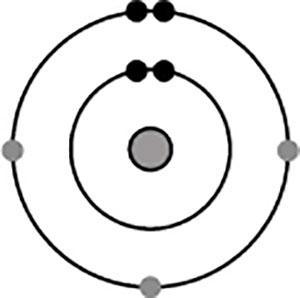
Answers
Answer:
The answer would be 15, letter D.
Explanation:
Since it has 5 electrons in its outermost shell, it will be located in group 15 in the periodic table.
The electron configuration of an atom of an element for the electrons in the s and p orbitals 15th group number of the element in the periodic table option D is correct.
What is electronic configuration of 15th group?Tha common electronic configuration of 15th group elements in ns2 np3, Group 15 elements in the modern periodic table are known as the pnictogens which means suffocation in Greek language.
This group consists of nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi).
The group 15 elements consist of five valence electrons. Due to this the elements can either lose five electrons or gain three electrons in order to attain the stable configuration. The general electronic configuration of nitrogen family is ns 2 np 3.
therefore , because of ns2 np3 option D is correct.
Learn more about electronic configuration , here :
https://brainly.com/question/13497372
#SPJ2
g a 0.1599 gram sample containing an unknown amount of chloride is titrated with a 0.7890 m silver nitrate solution. 30.83 ml of the silver nitrate solution was required to reach the end point of the titration. what is the mass percent of chloride in the original sample?
Answers
To determine the mass percent of chloride in the original sample, we need to first calculate the amount of chloride present in the sample. We can do this by using the balanced equation for the reaction between chloride and silver nitrate:
AgNO3 + Cl- → AgCl + NO3-
First, we need to calculate the number of moles of silver nitrate used in the titration:
moles of AgNO3 = volume (L) x molarity (M) = 0.03083 L x 0.7890 M = 0.02439 moles
Next, we can use the balanced equation to find the number of moles of chloride:
moles of Cl- = moles of AgNO3 = 0.02439 moles
Finally, we can use the number of moles of chloride to calculate the mass of chloride in the sample:
mass of Cl- = moles of Cl- x molar mass of Cl- = 0.02439 moles x 35.5 g/mol = 0.868 g
To find the mass percent of chloride in the original sample, we divide the mass of chloride by the total mass of the sample and multiply by 100%.
mass percent of Cl- = (mass of Cl- / total mass of sample) x 100%
Since we don't know the total mass of the sample, we can use the mass of chloride we just calculated and the formula above to find the mass percent of chloride in the original sample.
mass percent of Cl- = (0.868 g / 0.1599 g) x 100% = 54.3%
So, the mass percent of chloride in the original sample is 54.3%.
To learn more about mass percent:
https://brainly.com/question/26150306
#SPJ4
the pH of a solution is 2.0. what is the [OH^-] concentration?