Answers
Explanation:
check the steps in the attached image
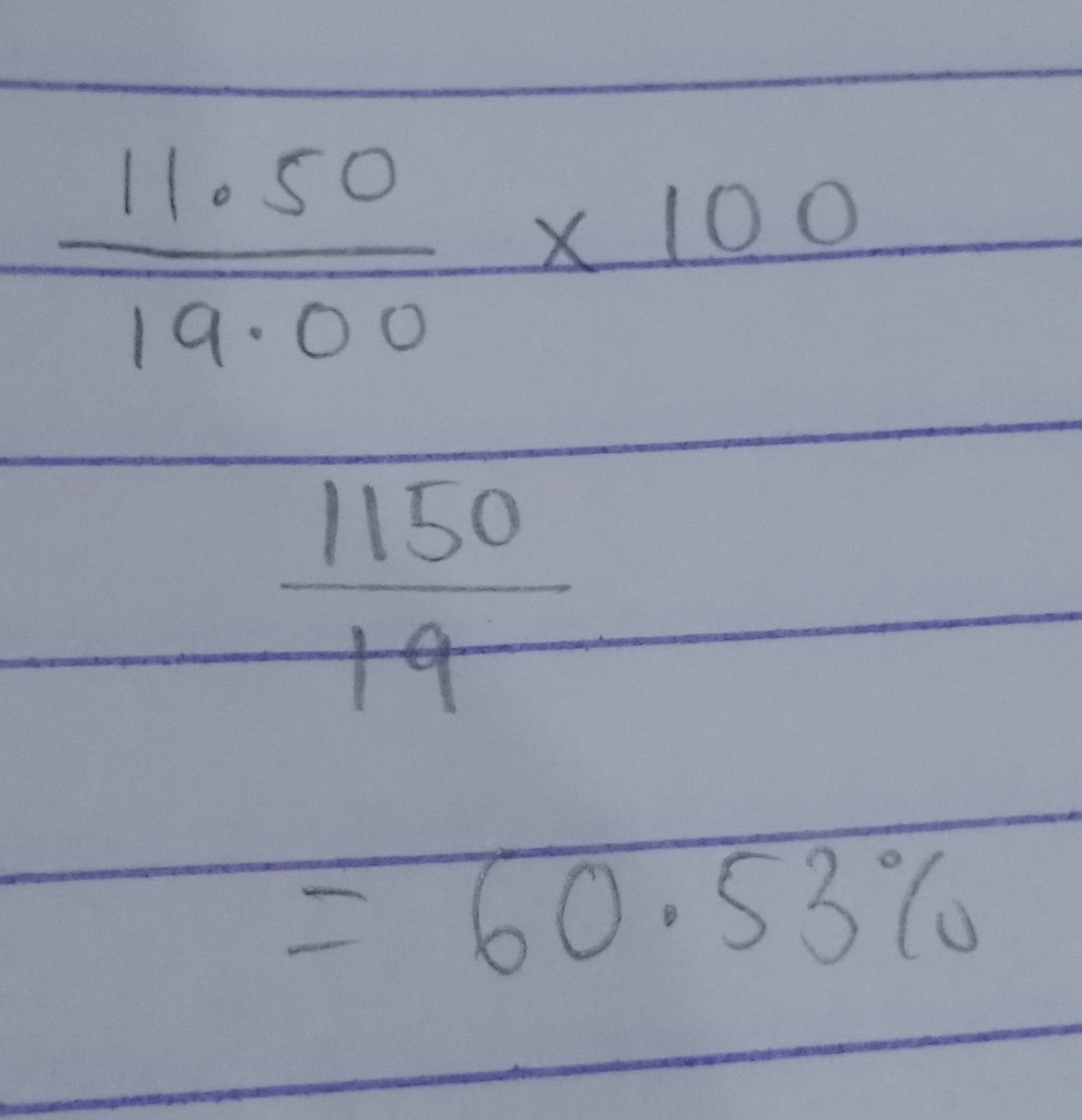
Related Questions
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25°C, diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine ΔHrxn for
C(diamond) → C(graphite)
with equations from the following list:
(1) C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ
(2) 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ
(3) C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ
(4) 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ
Answers
The enthalpy change of the reaction C(diamond) → C(graphite) is -2.9 kJ.
The given information is ΔHrxn for the reaction C(diamond) → C(graphite) can be calculated with the given equations:Equations: C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJThe required reaction can be obtained by adding the equations (1) and (4), as follows:C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g)Addition of the two equations (1) and (4) results in a reaction whose products are C(graphite) and CO2.
To get the final equation that involves only the required reactants and products, the equation (2) should be added, which consumes CO2 and produces O2, as shown below:C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ [eq. (1)] 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ [eq. (4)] 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ [eq. (2)] C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g) ΔHrxn=ΣΔHf(products)−ΣΔHf(reactants) ΔHrxn=[(3 mol CO2)(-393.5 kJ/mol) + (1 mol C(graphite))(0 kJ/mol)] − [(1 mol C(diamond))(0 kJ/mol) + (1 mol O2)(0 kJ/mol) + (2 mol CO(g))(−172.5 kJ/mol)] − [(2 mol CO2)(566.0 kJ/mol)] ΔHrxn=−2.9 kJ.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
the human population grew form 1 billion in the year 1800to blank billion in the year 200
Answers
The human population grew from 1 billion in the year 1800 to approximately 7.8 billion in the year 2021.
In the year 1800, the estimated global human population was around 1 billion. Over the next two centuries, significant advancements in technology, medicine, agriculture, and improved living conditions contributed to a rapid increase in population.
The growth rate of the human population began to accelerate in the 20th century. By the year 1927, the global population reached 2 billion. It took just 33 years for the population to double, reaching 4 billion in 1960. The population continued to grow at an unprecedented rate, with 6 billion people on Earth by the year 1999. As of 2021, the estimated global population stands at approximately 7.8 billion.
This remarkable growth in population can be attributed to several factors, including advancements in healthcare leading to reduced infant mortality rates, improved access to education and contraception, increased agricultural productivity, and overall socio-economic development.
It's important to note that population growth has not been uniform across all regions. Different countries and regions have experienced varying rates of population growth due to factors such as fertility rates, mortality rates, migration patterns, and government policies.
For more such questions on population visit:
https://brainly.com/question/30148263
#SPJ8
"Aqueous barium chloride reacts with aqueous sodium sulfate
to produce solid barium sulfate and aqueous sodium chloride."
Which chemical equation correctly translates the description
above?
Answers
Answer:
u should go to gathmath
Explanation:
it's alot better
6. The Grand Canyon is ______.
A. land surrounded by water
B. a dry, sandy area with no trees
C. a flat topped hill
D. a deep, narrow valley with steep sides
7. The large shapes of land that make up the earth are all called
______.
A. peninsulas
B. landforms
C. waterways
D. mountains
Answers
Answer:
6) D
7)A
Explanation:
B
It’s saying the answe has to be longer djdjenf
What is Na2Co3? How look like that's?
Answers
Sodium carbonate, often referred to as Na2CO3, is a chemical compound composed of atoms of sodium (Na), carbon (C) and oxygen (O).
It is also sometimes called washing soda or soda ash. At room temperature, sodium carbonate is a white, crystalline solid that is very soluble in water. According to the chemical formula of the sodium carbonate molecule, Na2CO3, each molecule consists of two sodium atoms (Na), one carbon atom (C) and three oxygen atoms (O). The atomic configuration in sodium carbonate is shown in the given diagram.
A trigonal planar arrangement is formed when the central carbon atom is bonded to three oxygen atoms. The structure of sodium carbonate is completed by two sodium atoms joined to oxygen atoms.
Learn more about Sodium carbonate, here:
https://brainly.com/question/31422792
#SPJ1

Copper coins turn green when exposed to water. What happens to this chemical reaction if the coins are heated?
1.The coins will turn green slower because chemical reactions slow down with higher temperatures
2.The coins will turn green faster because chemical reactions speed up with higher temperatures
3.The coins will turn green at the same rate as before because temperature has no affect on the speed of chemical reactions
4.The coins will not turn green, algae cannot survive in hot temperatures
Answers
Answer:
The coins will turn green faster because chemical reaction speed up with higher temperatures
Copper coins turn green when exposed to water because the coins will turn green faster because reaction speeds up with higher temperatures.
What is the rate of the chemical reaction?The rate of a chemical reaction can be defined as the speed at which the products are formed from the reactants. The rate of a reaction gives information about the time frame under which a reaction can be finished.
The rate of reaction can be described as the speed of a chemical reaction at which reactants are transformed into products. Some chemical reactions are generally instantaneous, while some reactions take time to reach the final equilibrium. Pressure increases the concentration of gaseous reactants resulting in an increase in the rate of reaction.
The rate of reaction will increase with an increase in the temperature of the reaction as the reactant molecules get more kinetic energy and increase the number of collisions.
Therefore, copper coins will turn green faster because reactions speed up with higher temperatures.
Learn more about the rate of reaction, here:
brainly.com/question/13571877
#SPJ2
answer please and thank u ok bye

Answers
It is the kind of interaction in which both organisms benefit from each other. *
Answers
Answer:
Mutualism
Explanation:
write down any four uses of solution
.I will mark him\her as brilliant
Answers
Answer:
because it will help u
because it will make u to understand the question very well
What is the coefficient for sodium chloride when this equation is balanced?
Answers
Answer:
To resolve this, we need to place the coefficient “2” in front of the sodium in the reactant, to give the equation shown below. 2 Na (s) + Cl 2 (g) → 2 NaCl (s) In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced.
Explanation:
scientific report on esterificatin
Answers
Esterification is a chemical reaction between an alcohol and a carboxylic acid that results in the formation of an ester and a molecule of water.
Write a scientific report on esterificationEsterification is an important class of organic reactions and is widely used in the synthesis of flavors, fragrances, and plastics. In this report, we will explore the fundamental principles and practical applications of esterification.
The experimental procedure involves the reaction between methanol and acetic acid to form methyl acetate and water. The reaction was carried out in a round-bottom flask equipped with a condenser and a thermometer. The reactants were mixed in stoichiometric amounts, and a small amount of sulfuric acid was added as a catalyst. The flask was heated using a hot plate and maintained at a constant temperature of 60°C.
Learn more about esterification:https://brainly.com/question/16010744
#SPJ1
Consider the following ionic compounds: CdCO3, Na2S, PbSO4, (NH4)3PO4, and Hg2Cl2. Which compounds will be soluble when added to water?
a. Na2S and (NH4)3PO4
b. Hg2Cl2
c. Hg2Cl2, Na2S, and (NH4)3PO4
d. CdCO3, PbSO4, and Hg2Cl2
Answers
Answer:
a. Na₂S and (NH₄)₃PO₄ .
Explanation:
To know which salts are soluble we need to remember some solubility rules. And apply these as follows:
* All compounds of group 1 (Li⁺, Na⁺, K⁺, etc.) are soluble, Na₂S is soluble.
* All ammonium salts are soluble,
(NH₄)₃PO₄ is soluble.* All Cl⁻, I⁻, Br⁻ are soluble except when are with Ag⁺, Hg₂²⁺, Pb²⁺, Hg₂Cl₂ is insoluble
* Carbonates are insoluble except those with group 1 or ammonium, CdCO₃ is insoluble
* All sulfates are soluble except those with Ca²⁺, Sr²⁺, Ba²⁺, Pb²⁺, Ag⁺. PbSO₄ is insoluble.
Right solution is:
a. Na₂S and (NH₄)₃PO₄Many ravines can be found in forested areas of the Cross Timbers ecoregion of Texas. What natural process most likely formed
these ravines?
OA. erosion by water during the rainy season
OB. erosion by wind during the dry season
OC. deposition by water during the rainy season
OD. deposition by wind during the dry season
Answers
Erosion by water during the rainy season can be a significant natural phenomenon. When it rains, water flows over the land, picking up soil particles and carrying them along with it.
What is water ?Water is a clear, colorless, odorless, and tasteless liquid that is essential for life. It is a chemical compound made up of two hydrogen atoms and one oxygen atom, with the chemical formula H2O. Water is the most abundant substance on Earth, covering approximately 71% of its surface.
Water is a unique substance because of its molecular structure, which allows it to exist in all three physical states - solid, liquid, and gas - at temperatures and pressures commonly found on Earth. It has a high heat capacity, meaning it can absorb and release large amounts of heat without changing temperature significantly. This property is important for regulating the Earth's climate and for maintaining the temperature of living organisms.
Water is essential for many biological processes, such as hydration, digestion, and metabolism. It also plays a crucial role in the Earth's water cycle, where it evaporates from bodies of water and plants, condenses into clouds, and falls back to the surface as precipitation.
To know more about Water visit :
https://brainly.com/question/28465561
#SPJ1
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
What would happen if Jupiter and Mars switched places? Please explain
Answers
A sample of water has a mass of 100.0 g. Calculate the amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C. Use the chart to complete the multiple steps required to arrive at the final answer. Type in your answers below using 3 digits.
q1 = ___ kJ
q2 = ___ kJ
q3 = ___ kJ
qtot = ___ kJ

Answers
The amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C is 83.8 kJ.
Quantity heat required to convert the ice to liquid
The total quantity of heat required is calculated as follows;
q(tot) = q1(to ice) + q2(fusion of ice) + q3(liquid)
q(tot) = mcΔθ₁ + mΔHf + mcΔθ₂
q(tot) = (100)(4.2)(0 - -45) + (334)(100) + (100)(4.2)(75 - 0)
q(tot) = 18,900 J + 33,400 J + 31,500 J
q(tot) = 18.900 kJ + 33.400 kJ + 31.500 kJ
q(tot) = 83,800 J = 83.8 kJ
Thus, the amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C is 83.8 kJ.
Learn more about quantity of heat here: https://brainly.com/question/13439286
#SPJ1
Answer:
q1 9.42
q2 226
q3 31.4
qtot 267
Explanation:
hope this help
0.030 mol He x22.4 L He
------------ = L He
1 Mol He

Answers
The balanced equation of the decomposition of hydrogen peroxide is as follows:
2 H₂O₂ ----> 2 H₂O + O₂0.030 mol He x 22.4 L He = 0.672 L He
What are the products of the decomposition of hydrogen peroxide?
Hydrogen peroxide is a compound of hydrogen and oxygen combined in a ratio of 1 : 1.
The products of the decomposition of hydrogen peroxide are water and oxygen gas. The equation of the decomposition of hydrogen peroxide is given below:
2 H₂O₂ ----> 2 H₂O + O₂
Learn more about the decomposition of hydrogen peroxide at: https://brainly.com/question/16896544
#SPJ1
Not a timed or graded assignment. Please refer to requirements circled in red, quick answer = amazing review :)

Answers
For this problem, we have to use Avogadro's number which is 6.022 x 10^(23) /mol. This number is telling us that there are 6.022 x 10^(23) atoms in 1 mole of a certain compound/element. The conversion would be:
\(0.30molesSO_2\cdot\frac{6.022\cdot10^{23}atoms}{1\text{ mol}}=1.8\cdot10^{23}atoms.\)The answer is that there are 1.8 x 10 ^(23) atoms of SO2 in 0.30 moles.
The 4-4-9 system is used to estimate the number of _______ in food. Question 9 options: A) kilojoules B) calories C) joules D) nutritional Calories
Answers
Answer: B) calories
Explanation:
The 4-4-9 system is used to estimate the number of calories in food. Under this system, it is assumed that one gram of Protein, one gram of Carbohydrates and one gram of Fats each contribute 4, 4 and 9 calories respectively to the caloric total.
This means that to find out the number of calories in food, multiply the total grams of proteins by 4, the total grams of carbohydrates by 4 and the total grams of Fats by 9 and then add up the results to find out the caloric contribution of Protein, Carbohydrates and Fats.
Answer:
It's not C calories i put that and missed it
Explanation:
Question 3
What part of a water molecule is the negatively charged side?
Answers
Answer:
The oxygen atom in water has a negative charge.
Definitions:Polar molecule: A molecule in which the centroid of the positive charges is different from the centroid of the negative charges.
Oxygen: A colorless, odorless, gaseous element constituting about one-fifth of the volume of the atmosphere and present in a combined state in nature. It is the supporter of combustion in air and was the standard of atomic, combining, and molecular weights until 1961, when carbon 12 became the new standard. Symbol: O; atomic weight: 15.9994; atomic number: 8; density: 1.4290 g/l at 0°C and 760mm pressure.
Water: A transparent, odorless, tasteless liquid, a compound of hydrogen and oxygen, H2O, freezing at 32°F or 0°C and boiling at 212°F or 100°C. that in more or less impure state constitutes rain, oceans, lakes, rivers, etc.: it contains 11.188 percent hydrogen and 88.812 percent oxygen, by weight.
Hydrogen: A colorless, odorless, flammable gas that combines chemically with oxygen to form water: the lightest of the known element. Symbol: H; atomic weight: 1.00797; atomic number: 1; density: 0.0899 g/l at 0°C and 760 mm pressure.
Atom: Am atom is the smallest constituent particle of a chemical element which has the properties of that element. They re comprised of at least an electron and a portion, as is the case for Hydrogen. Atoms of all other elements however, contain at least one neutron.
Proton: A positively charged elementary particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable baryon, having a charge equal in magnitude to that of the electron, a spin of 1/2, and a mass of 1.673 × 10-27kg. Symbol: P.
Electron: An elementary particle that is a fundamental constituent of matter, having a negative charge of 1.602 × 10-19 coulombs, ha mass of 9.108 × 10-31 kilograms, and spin of 1/23, and existing independently or as the component outside the nucleus of an atom.
Neutron: An elementary particle having no charge, mass slightly greater than that of a proton, and spin of 1/2: a constituent of the nuclei of all atoms except those of hydrogen. Symbol: n.
What is a negative charge?A object has a negative charge when it consists of more electrons than protons.
With a partial positive charge on 2 Hs and a partial negative charge on oxygen, water molecules are polar molecules.
Actually, two hydrogen atoms and the core oxygen atom of water are covalently connected. Due to its greater electronegative nature than hydrogen, oxygen pulls the bound electron pair in the middle of the atom toward it.
When illustrating higher electron densities over oxygen, partial negative charge is used, whereas partial positive charge is used to illustrate lower densities over hydrogen atoms.
#SPJ2
Plssss help ASAP I really need help with this y’all I’m bad at science

Answers
A weather balloon is filled with 28.6 L helium at sea
level where the pressure is 1.00 atm at 20.0 °C. The
balloon bursts after ascending until the pressure is 26.0
torr at -50.0 °C. Determine the volume (in L) at which
the balloon bursts.
Answers
The volume in which the weather ballon bursts is 640.21L.
How to calculate volume?The volume of a gas given the temperature and pressure can be calculated using the combined gas law as follows:
PaVa/Ta = PbVb/Tb
Where;
Pa, Va and Ta = initial pressure, volume and temperature respectivelyPb, Vb and Tb = final pressure, volume and temperature respectivelyAccording to this question, a weather balloon is filled with 28.6 L helium at sea level where the pressure is 1.00 atm at 20.0 °C.
1 × 28.6/293 = 0.034 × Vb/223
0.0976109215 × 223 = 0.034Vb
Vb = 21.767 ÷ 0.034
Vb = 640.21L
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
The particles of H2O are arranged differently in each state. In which state are particles fathers apart?
Answers
The three common states of matter are solid, liquid and gas. The intermolecular forces present between the particles in each state will be different. It is in gaseous state H₂O particles are fathers apart.
What is gaseous state?The gaseous state is the state of matter in which the intermolecular force of attraction is low than the liquid. The particles have a larger distance between the particles. The gaseous state of water is known as water vapor.
In gaseous state, the molecules have high kinetic energy and small intermolecular forces. Here the molecules can move randomly and the gas particles are highly compressible. They have lower density than solids and liquids.
The gaseous particles possess the property called diffusion. They have high volume and expand in the container they are placed. Here the particles move at higher speeds in all directions and hit each other.
Thus in gaseous form water has more freedom.
To know more about states of matter, visit;
https://brainly.com/question/4893275
#SPJ9
hurry i need answer rn pls help dont be a goofy
Using Figure 25-2, what type of star is Betelgeuse? 100 POINTS!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!111111111111111111111111111111111111111111111111111111111111111111111111111111111
Answers
Answer:
M2Lab/ supergiant star
Explanation:
Answer:
A super giant
Explanation:
When a solution containing 1.4000 g of Ba(NO3)2 and 2.4000 g of HSO3NH2 is boiled, a precipitate forms. One possible identity for this precipitate is Ba(SO3NH2)2.
a) Determine the limiting reagent for the balanced reaction, and then calculate the theoretical yield in moles.
b) Calculate the experimental molar mass if 1.6925 g of product were formed.
Answers
Answer:
See explanation for detailed solution
Explanation:
The balanced reaction equation is Ba(NO3)2 + 2HSO3NH2 → Ba(SO3NH2)2 + 2HNO3
Number of moles of Ba(NO3)2 = 1.4 g/ 261.337 g/mol = 5.36 × 10^-3 moles
From the reaction equation;
1 mole of Ba(NO3)2 yields 1 mole of Ba(SO3NH2)2
5.36 × 10^-3 moles of Ba(NO3)2 yields 5.36 × 10^-3 moles of Ba(SO3NH2)2
For HSO3NH2
Number of moles = 2.4g/97.10 g/mol =0.0247 moles
2 moles of HSO3NH2 yields 1 mole of Ba(SO3NH2)2
0.0247 moles of HSO3NH2 yields 0.0247 ×1/2 = 0.0137 moles
Hence, Ba(NO3)2 is the limiting reactant
The theoretical yield of Ba(SO3NH2)2 is 5.36 × 10^-3 moles × 329.4986 g/mol = 1.766 g
b)
Number of moles = mass/ molar mass
Molar mass = mass/ number of moles
Molar mass = 1.6925 g/5.36 × 10^-3 moles = 315.76 g
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
23.1 G Of HCI (a Strong Acid) Is Added To Water To Make 1250 ML Of Solution.
Answers
Answer:
[H₃O⁺] = 0.0507 M
pH = 1.29
Explanation:
23.1 g of HCI (a strong acid) is added to water to make 1250 mL of solution. Calculate [H₃O⁺] and pH of the solution.
Step 1: Given data
Mass of HCl (solute): 23.1 gVolume of solution: 1250 mLStep 2: Calculate the concentration of HCl
We will use the following expression.
[HCl] = mass HCl / molar mass HCl × liters of solution
[HCl] = 2.31 g / 36.46 g/mol × 1.250 L
[HCl] = 0.0507 M
Step 3: Calculate the concentration of H₃O⁺
HCl is a strong acid according to the following equation.
HCl(aq) + H₂O(l) ⇒ H₃O⁺(aq) + Cl⁻(aq)
The molar ratio of HCl to H₃O⁺ is 1:1. Then, [H₃O⁺] = 0.0507 M.
Step 4: Calculate the pH of the solution
We will use the following expression.
pH = -log [H₃O⁺]
pH = -log 0.0507
pH = 1.29
What are two types of fibres obtained from the fleece of a sheep? Which one is used to make wool?
Answers
Answer:
Answer: The two types of fibres obtained from the fleece of a sheep are beard hair, which are coarse and fine, and soft under hair, which grow near the skin. The under hair are used to make wool.
Explanation:
mark brainly please!
(I didn't copy the person above me! I just realized we had the same answer.)
10. For the reaction
H₂(g) + O₂(g) → H₂O(l)
H=-286 kJ/mol
What is the enthalpy change when 10.4 mol of hydrogen gas reacts with excess oxygen?
a. 27.5 kJ
b.-27.5 kJ
c. 3.64 x 10-2 J
d. -2.97 × 10³ J
e. -1.48 x 10³ J
Answers
I need the answer asap

Answers
Answer:
i really don't know
Explanation:
lol