Calculate the mass of iron that releases 2432 J of energy as its temperture rises from 25. 0 degrees * C to 87. 0 degrees * C. (The specific heat of iron is 0. 448 J/g^ C)
Answers
To solve this problem, we can use the formula:
q = m * c * ΔT
where q is the heat energy absorbed or released, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
We know that the heat energy released by the iron is 2432 J, the specific heat capacity of iron is 0.448 J/g°C, the initial temperature of the iron is 25.0°C, and the final temperature of the iron is 87.0°C.
The mass of iron that releases 2432 J of energy as its temperature rises from 25.0°C to 87.0°C is 96.2 g.
Substituting the values in the formula, we get:
2432 J = m * 0.448 J/g°C * (87.0°C - 25.0°C)
Simplifying the equation, we get:
m = 2432 J / (0.448 J/g°C * 62.0°C)
m = 96.2 g
Therefore, the mass of iron that releases 2432 J of energy as its temperature rises from 25.0°C to 87.0°C is 96.2 g.
To know more about temperature rise refer here:
https://brainly.com/question/2006890
#SPJ11
Related Questions
Question 1
1 pts
How many grams of sodium is contained in the final container when you dispense 564.2 mL of a
5.72 M sodium chloride solution into a beaker?
The atomic mass of sodium is 22.99 amu
The atomic mass of chlorine is 35.45 amu
Write your answer without units.
Next
Answers
Therefore, there are 73.3 grams of sodium in the final container.
Is sodium chloride acidic or basic?Sodium chloride (NaCl) is a neutral compound, meaning it is neither acidic nor basic. It is a salt formed by the combination of sodium (Na+) and chloride (Cl-) ions, which have a neutral charge and therefore do not affect the pH of a solution.
Firstly, the number of moles of sodium in the solution will be:
n = C * V = 5.72 M * 564.2 mL = 3.21 moles
Next, we convert the number of moles of sodium to grams:
mass = n * atomic mass = 3.21 moles * 22.99 amu/mole = 73.3 grams
To know more about sodium visit :-
brainly.com/question/29327783
#SPJ1
region a curve, surface, or solid?
Answers
The region can be a curve, surface, or solid. The term region refers to a specific area of space that can be expressed as a curve, surface, or solid.
A curve is a line that follows a path through space. A surface is a two-dimensional area that can be defined by an equation or a set of coordinates.
A solid is a three-dimensional object that has a length, width, height, firm, hard, or compact substance: solid ground. having relative firmness, coherence of particles, or persistence of form, as matter that is not liquid or gaseous
Therefore, the region can be any of the three, depending on how it is defined and expressed.
Read more about Space at https://brainly.com/question/27237286
#SPJ11
A gas sample was produced in the laboratory. The gas was determined to be more dense than air (which is mostly composed of nitrogen). What is the identification of the gas? a)Hydrogen b)Neon c)Methane (CH_4) d)Carbon Dioxide
Answers
The correct option is (d) Carbon Dioxide.
Explanation:
The density of air is around 1.2 g/L, which means that any gas with a density above this value is more dense than air.
Carbon dioxide has a density of approximately 1.98 g/L, which is considerably more dense than air (composed of nitrogen and oxygen).
As a result, if a gas sample is determined to be more dense than air, it is likely to be carbon dioxide (CO2), which has a molecular weight of 44 g/mol.
Carbon dioxide is produced in the laboratory by many chemical reactions and is commonly employed in the food and beverage industries, such as carbonating soda and beer.
To know more about chemical reactions visit;
https://brainly.com/question/29762834
#SPJ11
Here is a sample graph from Lesson 4.2 - Can you determine the general relationship between the percentage yield of ammonia and pressure?
Which temperature was the most beneficial for this experiment?
How many different “systems” were tested here?
Here’s a tough one -
Which was held “constant” during the test run? Select one.
a. The percentage yield of ammonia
b. Pressure
c. Temperature
d. All were constant
e. None were constant
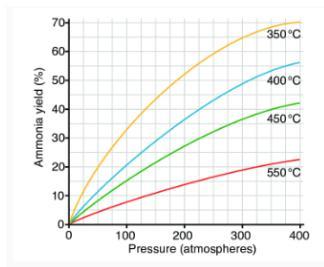
Answers
Option D is correct All remained the same Here, a variety of "systems" were put to the test.
Throughout an experiment, the control variable is constant. In an experiment, the independent variable is changed in an effort to change the experiment's dependent variable. In order to ensure that the effects on the dependent variable are definitely caused by changes in the independent variable, the control variable is kept constant.
The gas constant can be expressed using the values and units listed below. The values of the gas constant R are typically listed under "Physical Constants" in textbooks and handbooks because it is a constant that applies to all gases without exception. When the temperature remains constant, the pressure of a gas is inversely proportional to its volume. When the temperature is constant, the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariette's law.
Learn more about variable from here;
https://brainly.com/question/30763805
#SPJ1
What is the relationship between the enthalpy (AH) and entropy (AS) of a
reaction that is never spontaneous?
OA. +AH,-AS
OB. -AH, +AS
OC. -AH-AS
OD. +AH, +AS
SUBMIT
Answers
The relationship between the enthalpy (ΔH) and entropy (ΔS) of a reaction that is never spontaneous is -ΔH, +ΔS option - B is correct answer.
A spontaneous reaction is what?When a reaction occurs spontaneously, the system doesn't require any additional energy input because the change in free energy is negative.
When the enthalpy change is negative and the entropy change is positive, the reaction is always spontaneous.
The free energy change is always positive and the reaction is never spontaneous if the reaction is endothermic (H positive) and the entropy change S is negative (less disorder).
Although a spontaneous reaction may result in an increase or decrease in entropy or enthalpy, it will always result in a decrease in free energy, which is a negative G.
To know more about enthalpy visit:
https://brainly.com/question/13996238
#SPJ1
Which of the following elements could be prepared by electrolysis of the aqueous solution shown?
Multiple Choice
Sodium from Na3PO4(aq)
Sulfur from K2S04(ed)
Oxygen from H2SO4(aq)
Potassium from KCl(aq)
Nitrogen from AgNO3(aq)
Answers
Sodium from Na3PO4(aq) could be prepared by electrolysis of the aqueous solution shown. Based on the provided options, the element that could be prepared by electrolysis of the aqueous solution shown
Potassium from KCl(aq)
Here's why:
- Sodium from Na3PO4(aq) and Nitrogen from AgNO3(aq) are not possible because these ions are more stable in solution than undergoing electrolysis.
- Sulfur from K2S04(ed) is not valid as the compound should be K2SO4(aq) and even then, it would produce oxygen at the anode instead of sulfur.
- Oxygen from H2SO4(aq) can be prepared through electrolysis, but this is not an element directly obtained from the compound.
Potassium from KCl(aq) can be prepared by electrolysis. During this process, K+ ions are reduced to potassium metal at the cathode, and Cl- ions are oxidized to chlorine gas at the anode.
Visit here to learn more about electrolysis:
brainly.com/question/12054569
#SPJ11
What non-metal has less than 4 valence electrons (Outmost cells electrons)?
Answers
Correct Answer: Boron family
HCL is pure covalent compound but soluble in solvent water why
Answers
Answer:
HCl is a polar covalent compound, because of electronegativity difference between Cl(3.5) and hydrogen (2). Hence in this way, the bond between HCl breaks and they formed ions in the polar solvent like water .
a gas has a mass of 3175 g and takes up enough space to fill a room that is 2.00 m x 2.00 m x 5.00 m determined what the gas is in g/mL
Answers
To determine the gas in g/mL, we need to calculate the volume of the gas first. We can use the formula for the volume of a rectangular room to find the volume of the room:
Volume = length x width x height = 2.00 m x 2.00 m x 5.00 m = 20.00 m³
Next, we need to convert the mass of the gas from grams to kilograms, since density is usually expressed in units of kg/m³. We can do this by dividing the mass by 1000:
Mass = 3175 g ÷ 1000 = 3.175 kg
Finally, we can calculate the density of the gas using the formula:
Density = Mass ÷ Volume
Density = 3.175 kg ÷ 20.00 m³ = 0.1588 kg/m
To convert the density from kg/m³ to g/mL, we need to multiply by 1000 and then divide by 1000:
Density = 0.1588 kg/m³x 1000 g/kg ÷ 1000 mL/L = 0.1588 g/mL
Therefore, the gas has a density of 0.1588 g/mL. Without additional information, we cannot determine the identity of the gas.
To know more about density, visit :
https://brainly.com/question/29775886
#SPJ1
890J of heat are applied to a piece of aluminum, causing a 4.6°C increase in its temperature. The specific heat of aluminum is 0.902 J/g°C. What is the mass of aluminum?
Question 12 options:
175g
3,693g
214g
0.00220g
Answers
Answer:
214
Explanation:
Q=mco
Q=heat
M is mass
C is specific heat
O is increased in temperature
A solution is tested and found to have a pH of 1.
The solution is
A.
weakly basic.
B.
strongly basic.
C.
strongly acidic.
Answers
Answer: strongly acidic
Explanation: a strongly acidic solution has a pH between one and four
In this lab, you will observe the properties of oil,
cornstarch, salt, and sodium bicarbonate. You will
use the properties to classify the substances as
ionic and covalent. Restate this goal in the form of
an investigative question.
Answers
The ionic compounds are salt and sodium bicarbonate. The covalent compounds are oil and cornstarch.
The ionic or covalent bond in the structure can be assessed in the laboratory by dissolving the compounds in the water.
The ionic compounds on dissolution will disintegrate into positive and negative ions.
The oil dissolving in the water will float onto it, thus the compound did not dissociate and has a covalent bond in the structure.
The cornstarch is a carbohydrate moiety and on dissolving in the water will be unable to disintegrate. The compound is composed of a covalent bond.
The salt on dissolving in water completely dissociates and becomes soluble. The compound disintegrates into the constituent ions. Thus, salt is an ionic compound.
Sodium bicarbonate is also an ionic compound, as it dissociates into the water.
The investigation question will be: Which of the following compound has an ionic and covalent bond?
a 1 liter solution contains 0.245 m nitrous acid and 0.327 m potassium nitrite. addition of 0.123 moles of potassium hydroxide will:
Answers
Addition of 0.123 moles of potassium hydroxide will increase the PH of the solution by several units .
Why the addition of 0.124 moles of potassium hydroxide will increase the PH by some units?PH is the basic term in chemistry which signifies the hydrogen ion concentration in a given solution.Here is given in a 1 liter solution is 0.245 molal nitrous acid and 0.327 molal potassium nitrite.And the question is asked of effect in the solution after the addition of 0.123 moles of potassium hydroxide in the same solution.See nitrous acid that is KNO2 is a strong acid , and KOH is a strong base so it will completely ionize in the solution.The k+ component of the KOH will react with nitrous that is NO2 component and will form KNO2 which is a weak base .So being basic in nature it will slightly increase the PH of the solution where in it will react.To know more about solution visit:
https://brainly.com/question/1616939
#SPJ4
What mass to the nearest tenth of a gram, is needed to balance this chemical
reaction equation?
16AI
+
35
-
8AIS
8
2 3
+
7.4 g
24.5 g
24.5 g
o
17.1 g
131.99
None of the above
Answers
none of the above. is the answer
Why is it important to turn off the power supply when the loading buffer gets toward the bottom of the gel?.
Answers
At this condition, it is necessary to to stop the current as the molecules may pass through the gel if the current is allowed to continue even after the loading buffer gets towards the bottom of the gel.
A narrow dye front is seen when the gel percentage is low, whereas a diffuse dye front is seen when the acrylamide percentage is large in the gel. To prevent a circuit, the power should be turned off before removing the gels and then the cords. When an electric current is sent through a gel, charged molecules move throughout the substance. One end of the gel receives a positive charge, and the other receives a negative charge due to the application of an electric current across the gel. Migration describes how charged molecules migrate. To the opposing charge, molecules move. In the sample wells at the top of the gel, protein samples are introduced.
Learn more about molecules here:
https://brainly.com/question/25617532
#SPJ4
Determine whether HI can dissolve each metal sample. If it can, write a balanced chemical reaction showing how the metal dissolves in HI and determine the minimum volume of 3.5MHI required to completely dissolve the sample.
a. 2.15gAl.
b. 4.85gCu.
c. 2.42gAg.
Answers
A minimum of 25.6 mL of 3.5 M HI is needed to thoroughly dissolve 2.42 g of silver.
Which metals will HCl dissolve?The less active metals, including zinc and magnesium, are easily dissolved by hydrochloric acid. Iron, copper, and other harder metals are less easily or completely disintegrated by it. While hydrochloric acid won't dissolve some metals, other chemicals, such nitric acid, will.
The chemical equation for the reaction of copper with HI is balanced as follows:
Cu(s) + 2HI(aq) → CuI2(aq) + H2(g)
The following formula can be used to determine the minimum volume of 3.5 M HI needed to completely dissolve 4.85 g Cu:
Moles of Cu = 4.85 g / 63.55 g/mol = 0.0763 mol
Moles of HI required = 2 × moles of Cu = 0.1526 mol
Volume of 3.5 M HI required = moles of HI required / 3.5 M = 0.0436 L or 43.6 mL
Therefore, 43.6 mL of 3.5 M HI is the lowest amount needed to thoroughly dissolve 4.85 g of copper. The chemical equation for the silver-HI reaction is balanced as follows:
2Ag(s) + 4HI(aq) → 2AgI(s) + 2H2(g)
The following formula can be used to determine the minimum volume of 3.5 M HI needed to completely dissolve 2.42 g of Ag:
Moles of Ag = 2.42 g / 107.87 g/mol = 0.0224 mol
Moles of HI required = 4 × moles of Ag = 0.0896 mol
Volume of 3.5 M HI required = moles of HI required / 3.5 M = 0.0256 L or 25.6 mL
To know more about silver visit:-
https://brainly.com/question/6434391
#SPJ1
Create a hypothesis for the following problem:You are planting a garden. Create a hypothesis about how water will affect plant growth.
Answers
Answer:
I hypothesize that if I water the garden reguarlary, making sure that the plants are not dry, that the plants will thrive. However, if you do not water them, the plants will die.
Explanation:
Hope this helps! :)
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pressure, how would the absolute temperature of the gas be affected?
A gas at 300 K and 4.0 atm is moved to a new location with a temperature of 250 K. The volume changes from 5.5 L to 2.0 L. What is the pressure of the gas at the new location?
What is the partial pressure of 0.50 mol Ne gas combined with 1.20 mol Kr gas at a final pressure of 730 torr?
Answers
**Check for proof photos at the bottom.**
**Answers are in bold.**
__________________________________________________________
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pressure, how would the absolute temperature of the gas be affected?
✔ it would decrease threefold
A gas at 300 K and 4.0 atm is moved to a new location with a temperature of 250 K. The volume changes from 5.5 L to 2.0 L. What is the pressure of the gas at the new location?
✔ 9.2 atm
What is the partial pressure of 0.50 mol Ne gas combined with 1.20 mol Kr gas at a final pressure of 730 torr?
✔ 215 torr
__________________________________________________________
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
✔ it is decreased to one third of its original volume
Why might a rubber raft burst if it is left in the sun on a summer day?
✔ temperature increases, causing interior pressure to increase
__________________________________________________________
Complete the table by filling in the missing information.
✔ pressure, volume A
✔ temperature, moles of gas B
✔ Charles’s law C
✔ V = kT D
✔ temperature, pressure E
✔ P = kT F
✔ number of moles only G
__________________________________________________________
Match one graph shown at right to each of the gas laws named below.
Charles’s law
✔ d
Gay-Lussac’s law
✔ b
Boyle’s law
✔ e
__________________________________________________________
Left answers:
1.31 atm A
0.44 atm B
Right answers:
✔ decreases it
✔ decreases it
0.12 atm C
__________________________________________________________
Explanation:When decreasing a volume while maintaining the same pressure, the temperature will have to decrease. When volume decreases, particles get squished, increasing pressure. But when temperature decreases, particles move slower, decreasing pressure. The lowered temperature decreases pressure, but the decreased volume increases pressure, so pressure is able to stay constant.
About the 3 main gas laws covered in Edge:
Charles's law says that under a constant pressure, when temperature increases, the volume increases, and vice versa. The equation for this law is V₁/T₁=V₂/T₂.Gay-Lussac’s law says that under a constant volume, when temperature increases, the pressure increases, and vice versa. The equation for this law is P₁/T₁=P₂/T₂.Boyle’s law says that under a constant temperature, when volume decreases, pressure increases, and vice versa. The equation for this law is P₁V₁=P₂V₂.Note that P=pressure, V=volume, and T=temperature. When given a problem, plug in the given values into the correct spots and solve for the unknown value.
Here are photos of Edge. I hope this made your day a little easier.





What is the difference between distilled water and purified water.
Answers
Answer:
Distilled water is a type of purified water that has had both contaminants and minerals removed. Purified water has had chemicals and contaminants removed, but it may still contain minerals. ... Distillation boils the water, and then condenses the steam back into a liquid to remove impurities and minerals.
Calculate the number of moles water produced by the complete reaction of
109 g of hydrogen. 99
Answers
The number of moles water produced by the complete reaction of
109 g of hydrogen. 99 is given below:
What is moles ?Moles in chemistry are a unit of measurement used to quantify the amount of substance in a given sample. It is defined as the amount of a substance that contains the same number of particles as there are atoms in 12 grams of Carbon-12. This is known as Avogadro's number and is equal to 6.02 x 10^23. The mole allows scientists to calculate the mass, volume, and number of atoms or molecules in a given sample. It is a very useful tool for measuring the amount of a substance, as it takes into account the fact that different elements and compounds have different molecular weights. The mole is also used in equations to determine the mass, volume, and concentration of a substance, as well as the number of molecules in a given sample.
The equation for the complete reaction of hydrogen is:
2H2 + O2 → 2H2O
Since one mole of hydrogen reacts with one mole of oxygen to produce two moles of water, we can calculate the number of moles of water produced by the complete reaction of 109 g of hydrogen.
Using the molar mass of hydrogen (2.016 g/mol), we can calculate the number of moles of hydrogen present:
Moles of hydrogen = (109 g H2) / (2.016 g/mol H2)
Moles of hydrogen = 53.9 mol H2
Since one mole of hydrogen reacts with one mole of oxygen to produce two moles of water, the number of moles of water produced by the complete reaction of 109 g of hydrogen is:
Moles of water = (53.9 mol H2) x (2 mol H2O/1 mol H2)
Moles of water = 107.8 mol H2O
To learn more about moles
https://brainly.com/question/15356425
#SPJ1
The process of changing a gas to a liquid is known as _____.
sublimation
precipitation
evaporation
condensation
Answers
Answer:
Condensation is the process.
An atom can be compared to our solar system because: WILL MAKE BRAINLIEST!
The nucleus gives off light like the sun.
Both electrons and planets move quickly.
Electrons orbit the nucleus like planets orbit the sun.
All of the above.
Answers
Answer:
uh i think its D All of the above
Explanation:
sorry if its wrong
Answer:
all of the above
Explanation:
The nucleus is in the middle and it does glow from the energy it has, the electros move in an orbit around the nucleus just like the planets so that would be correct and the electrons do move fast.
Which keystone species feed on other animals and prevent them from damaging the ecosystem?
Answers
A teacher asks a student to write the chemical equation for photosynthesis. The student's response is shown below.
CO2+H2O→C6H12O6+O2
The equation is not balanced correctly. Which of these is a balanced equation for photosynthesis?

Answers
Answer:
A
Explanation:
Trust me bro :) I just know it
What is the oxidation number of carbon in the compound carbon dioxide, CO2?
A) +3
B)+2
C)-3
D)+4
Answers
Answer:
the oxidation number is 4
Does ionic bonds dissolve in water?
Answers
Answer:
Yes ionic bonds do dissolve in water
Explanation:
Water is a polar solvent and has two poles one negative and another positive. The two poles of water have strong forces of attraction towards other charged ions, due to this water breaks the ionic bond by hydrogen bond formation. Therefore, ionic bonds dissolve in water.
hope this helps
How many ions of silver can be found in 2.84 moles of silver phosphate?
Answers
Answer:
Ksp=[Ag+]^3[PO4^3-]
For every 1 mole silver phosphate dissolved, 3 moles Ag+ will be ionized so plug in 3x for [Ag+]. For [PO4^3-] only 1 mole will ionize so just 1x.
Ksp=[3x]^3[x]
Ksp=27x^4
x=1.82x10^-9.
Explanation:
hope this helps also im sorry if it is to conffusing hard to explain
(Look at the chart) You are trying to figure out the identity of a lump of yellow metal you found. You use a scale to determine that it has a mass of 50g.
You fill a container with 100mL.
What kind of metal is the lump you found?
LOOK AT THE CHART
-impossible to know
-gold
-copper
-silver

Answers
Explanation:
Volume of solid metal = 102.6ml - 100ml = 2.6ml.
Mass of solid metal = 50g.
Density = Mass / Volume = 50g / 2.6ml = 19.2g / cm³.
Also from our knowledge, only gold metal is yellowish here.
Hence the answer is gold metal.
What is the correct electron configuration of phosphorus (P)?
O A. 1s22s²2p³3s²3p³
B. 1s²2s²2p 3s²3p³
O C. 1s²2s²2p62d5
OD. 1s²2s22p63s¹3p4
Answers
Answer:
A
Explanation:
Phosphorus has an electron configuration of 1s2 2s2 2p6 3s2 3p3. Another way to write that is [Ne] 3s2 3p3. The [Ne] represents the fact that the beginning of phosphorus' electron configuration is the same as Neon's. so if you add all the superscripts they will give you 15. we didn't use choice d because we have to make the last p halffilled(3, half of the total electron held in p shell ) when we make "s shell 1 " not make it 4 because we are transfering that 1 electron .but nevertheless the correct answer is A.
Can two (or more) types of matter occupy the same space at the same time?
Answers
The general properties of matter result from its relationship with mass and space. ... Because it occupies space, all matter has volume and impenetrability, since two objects cannot occupy the same space simultaneously.