Cause and Effect. Unbalanced forces acting on a Nebula result in___
a. A constant linear motion
b. Equilibrium of the nebula
c. A change in its motion
Answers
Unbalanced forces acting on a Nebula result in a change in its motion.(C)
When unbalanced forces act on a nebula, they disrupt its equilibrium and cause a change in its motion. This is due to Newton's First Law of Motion, which states that an object at rest or in constant linear motion will continue in that state unless acted upon by an unbalanced force.
In the case of a nebula, the unbalanced forces may come from nearby stellar explosions, passing stars, or gravitational interactions.
These forces can cause parts of the nebula to compress and collapse, initiating the formation of new stars and planetary systems. As a result, the motion of the nebula changes over time as it evolves and develops under the influence of these forces.(C)
To know more about Newton's First Law of Motion click on below link:
https://brainly.com/question/29775827#
#SPJ11
Related Questions
if 1.35 g of aluminum occupies 0.500 cm^3. what is the density of aluminum
Answers
Answer:
2.7
Explanation:
Density= mass/volume
= 1.35/0.500
= 2.7
What is the concentration of H₂(g), in parts per million, in a solution that contains 0.0001 g of H2(g) dissolved in 100. g of H₂O(l)?
Answers
Answer:
1 ppm :)
Explanation: i was guessing and 1 ppm was the answer :)
Un elemento tiene 3 orbítales desapareados y presenta 3 niveles de
energía. ¿A qué grupo y período peretenece?
a) IIIA, 3 b) IIIB, 3 c) VIA, 3 d) VB, 3 e) VA, 3
Answers
Hello whats the answer to"What is 20 000 Hz In kHz" thanks this for my range of hearing homework
Answers
Answer:
=20kHz
.............................
why is vapor unlikely to behave as an ideal gas near the temperature at which the vapor would liquify?
Answers
Vapor unlikely to behave as an ideal gas near the temperature at which the vapor would liquify the intermolecular forces between the gas molecules become more significant as the temperature decreases.
What is an ideal gas?An ideal gas can be envisioned as a hypothetical gas consisting of a collection of randomly-dispersed, non-interacting particles with negligible volume.
The notion of an ideal gas proves valuable as it adheres to the principles of the ideal gas law, which is a simplified equation of state.
Furthermore, the behavior of an ideal gas lends itself to analysis through the lens of statistical mechanics.
Learn about ideal gas law here https://brainly.com/question/27870704
#SPJ4
can of soda (355 ml) has 40 g of high fructose corn syrup (mw = 180 g/mol). what is its molarity?
Answers
The molarity of a solution can be calculated using the formula:
Molarity = (amount of solute in moles) (volume of solution in liters)
In this case, the amount of solute is 40 g of high fructose corn syrup and the volume of solution is 355 ml or 0.355 L.
First, we need to convert the amount of solute from grams to moles. To do this, we can use the molecular weight (mw) of high fructose corn syrup, which is 180 g/mol:
Amount of solute in moles = (40 g) / (180 g/mol) = 0.222 mol
Now we can plug this value into the formula for molarity:
Molarity = (0.222 mol) / (0.355 L) = 0.625 M
Therefore, the molarity of the high fructose corn syrup in the can of soda is 0.625 M.
Read more about molarity here: https://brainly.in/question/1159016
#SPJ11
The molarity of a solution can be calculated using the formula:
Molarity = (amount of solute in moles) / (volume of solution in liters)
In this case, the amount of solute is 40 g of high fructose corn syrup and the volume of solution is 355 ml or 0.355 L.
First, we need to convert the amount of solute from grams to moles. To do this, we can use the molecular weight (mw) of high fructose corn syrup, which is 180 g/mol:
Amount of solute in moles = (40 g) / (180 g/mol) = 0.222 mol
Now we can plug this value into the formula for molarity:
Molarity = (0.222 mol) / (0.355 L) = 0.625 M
Therefore, the molarity of the high fructose corn syrup in the can of soda is 0.625 M.
Read more about molarity here:
https://brainly.in/question/1159016
#SPJ4
calculate the molarity of the solution in a flask that contains 2.50 moles of potassium sulfate in 125 mL of solution
Answers
Answer:
0.02 M
Explanation:
Molarity = moles/volume (L)
125 mL =0.125L
M = 2.5/0.125 = 0.02 M
what kind of reaction is represented by the equation ch4 2 o2 ® co2 2 h2o?
Answers
The reaction represented by the equation CH_4 + 2 O_2 → CO_2 + 2H_2O is a combustion reaction.
A combustion reaction is a type of chemical reaction in which a substance reacts with oxygen to produce heat and light. In this case, methane (CH_4) is reacting with oxygen (O_2) to produce carbon dioxide (CO_2) and water (H_2O).
Combustion reactions are typically exothermic, meaning they release energy in the form of heat and light. They are often accompanied by a flame and are commonly observed in processes such as burning of fuels, such as natural gas, gasoline, or wood.
In this specific reaction, methane (CH_4) is the fuel that undergoes combustion, combining with oxygen (O_2) to form carbon dioxide (CO_2) and water (H_2O). The coefficients in front of the molecules indicate the stoichiometric ratio, showing that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
Learn more about combustion reactions from this link:
https://brainly.com/question/31123826
#SPJ11
30)
A scientific theory is based on
A)
data collected over time.
B)
a general claim by scientist.
0
the best guess of a scientist.
D)
an idea that can only be proven true.
I think it’s (a)but, I’m not sure.
Answers
Answer:
D has to be based on facts so d would be the answer and idea that can only be proven true those are facts not hypothesis or guesses
Explanation:
based on a body of facts that have been repeatedly confirmed through observation and experiment. Such fact-supported theories are not "guesses" but reliable accounts of the real world."
calculate the heat (q) in kj released with combustion of methanol using 790 grams of water and the change in temperature is 9.1 degree celsius. the specific heat of water is 4.18 j/(g*c). report and round your answer the an integer.
Answers
To calculate the heat released during the combustion of methanol, you will need to know the heat of combustion of methanol, which is the amount of heat released when a given amount of methanol is burned in oxygen.
The heat of combustion of methanol is -726 kJ/mol.
To find the heat released in kJ using 790 grams of water and a temperature change of 9.1 degrees Celsius,
you will need to use the formula q = m * c * deltaT, where q is the heat released, m is the mass of the substance being heated (in this case, water), c is the specific heat of the substance, and deltaT is the change in temperature.
In this case, the heat released would be:
q = 790 g * 4.18 J/(g*C) * 9.1 C = 28,903.22 J.
To convert this to kJ, you would divide by 1000 to get 28.90322 kJ. Rounded to the nearest integer, the heat released would be 29 kJ.
Learn more about combustion of methanol:
https://brainly.com/question/12655480
#SPJ4
Which types of radioactive decay is shown in all of the equations in the image? What are two indicators of the type of decay occurring?

Answers
There are two signs of alpha decay. The matrix 4/2He is used in all of the equations to represent the emission of an alpha particle, which is a helium nucleus made up of two protons and two neutrons.
What transpires in each of the three primary forms of radioactive decay?The nucleus loses two protons during alpha decay. The nucleus either acquires or loses a proton during beta decay. There is no change in the proton count during gamma decay.
The nuclear equation illustrates what radioactive decay.Alpha decay can be described by a nuclear equation. If the total numbers of protons and neutrons on both sides of the arrow are equal, the equation is balanced. Alpha decay is one of the four types of radioactive decay.
To know more about alpha decay visit:-
https://brainly.com/question/14081292
#SPJ1
1. Ba(OH)2
What is the compound
Answers
Answer:
BARIUM HYDROXIDE IS THE COMPOUND
For molecules with only one central atom, how many lone pairs on the central atom guarantees molecular polarity?
Answers
Answer:
The answer is "1".
Explanation:
It is the lone pairs at, which the central atom provides the molecular and the polarity. It is only for substances with just a central atom, for which the molecules with only one central atom and only one of the solitary paired were required, that's why in this question the "1" is the correct answer.
in this short synthetic sequence, provide the organic structures of the missing reactant and the missing product.
Answers
The first step of the reaction, we have reaction between a nucleophile and an electrophile.
We can see that in the first step of the reaction, we have reaction between a nucleophile and an electrophile. In this case, the electrophile would have to be an alkyl halide which produces a carbocation as show in the image attached. What we have here is quite similar or like most of the organic reactions, this reaction occurs in a number of detailed or smaller steps and each step of the reaction is going to help to bring us closer to the end product of the entire steps of the reaction which is wat we target as we carry out the particular reaction.The second step involves the reduction of the alkyne with the use of a Lindlar catalyst. As such the reaction is poisoned and it stops at the alkyne stage rather than going on to obtain the alkane.
Learn more about electrophile here:
https://brainly.com/question/21773561
#SPJ4

How are protons and neutrons the same and how are they different?
Answers
Explanation:
Protons, Neutrons and Electrons are the three primary subatomic particles that form an atom.
Protons and Neutrons are large particles and are densely compacted into the nucleus of the atom. Protons are electrochemically positive in charge and the Neutrons are electrochemically neutral in charge.
Together the protons and Neutrons make up the mass of the atom.
Electrons are electrochemically negatively charged particles that move random around the nucleus. They have a relatively small mass compared to Protons and Neutrons. They are found in electron clouds that surround the nucleus and their movement and properties provide for the bonding characteristics of each atom.
in which of these compounds are there twice as many oxygen atoms as hydrogen atoms?

Answers
Answer:
G - H2SO4
Explanation:
two hydrogen atoms and 4 oxygen atoms
Select True or False for each statement: The solubility of gases in water decreases with increasing temperature Most solids are more soluble at higher temperature. Pressure has little effect on the solubility of liquids and solids because they are almost incompressible.
Answers
The solubility of a substance is influenced by many factors, including temperature, pressure, and the type of solvent used. Knowing the solubility of a substance can be useful in a variety of applications, such as:
The production of medicinesFood productsOther consumer goodsLearn more about solubility: https://brainly.com/question/23946616
#SPJ4
the molecule that provides the energy to drive endergonic reactions in the body is abbreviated
Answers
The molecule that provides the energy to drive endergonic reactions in the body is abbreviated as ATP, which stands for adenosine triphosphate. ATP is a high-energy molecule that serves as the primary source of energy for various cellular processes and reactions.
Endergonic reactions are those that require an input of energy to proceed. In biological systems, this energy is often provided by ATP. The ATP molecule is composed of a nitrogenous base (adenine), a sugar (ribose), and three phosphate groups. The energy stored in ATP is mainly found in the bonds between the phosphate groups.
When a cell needs energy for an endergonic reaction, ATP undergoes hydrolysis, a process in which a phosphate group is removed from the molecule, resulting in the formation of adenosine diphosphate (ADP) and an inorganic phosphate (Pi). This reaction releases the energy that can be utilized to power various cellular processes, such as muscle contraction, protein synthesis, and cellular transport.
Conversely, the energy released during exergonic reactions (reactions that release energy) can be harnessed to regenerate ATP from ADP and Pi. This continuous cycle of ATP hydrolysis and regeneration ensures that cells have a constant supply of energy to drive endergonic reactions and maintain various biological functions.
In summary, ATP is the key molecule that provides the energy required for endergonic reactions in the body. It acts as a universal energy currency, allowing cells to store, transfer, and utilize energy efficiently for a wide range of cellular processes.
learn more about adenosine triphosphate here: brainly.com/question/28431482
#SPJ11
briefly describe any example of an endothermic reaction and also mention the word equation
Answers
Explanation:
\(\huge{\underbrace{\overbrace{\mathfrak{\pink{Answer:}}}}}\)
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical processes can be endothermic as well – Ice cubes absorb heat energy from their surroundings and melt to form liquid water (no chemical bonds are broken or formed).
When a chemical bond is broken, it is usually accompanied by a release of energy. Similarly, the formation of chemical bonds requires an input of energy. The energy supplied/released can be of various forms (such as heat, light, and electricity). Endothermic reactions generally involve the formation of chemical bonds through the absorption of heat from the surroundings. On the other hand, exothermic reactions involve the release of heat energy generated from bond-breakage.
Endothermic Reaction Examples
Ammonium nitrate (NH4NO3), an important component in instant cold packs, dissociates into the ammonium cation (NH4+) and the nitrate anion (NO3–) when dissolved in water

What does each row (across) have in common?
Answers
catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (HNO3) on a commercial scale.
The products are produced at 1000°C (1273 K) and at at-
mospheric pressure.
4 NH; (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (1)
a. What volume of NO is produced in the reaction vessel
by the reaction of 0.500 mol O2?
b. What mass of H2O is produced by the reaction of 15.0 L
of NH3?
c. How many liters of O, must react to produce 35.5 L of
NO?
Answers
a. volume of NO : 41.785 L
b. mass of H2O : 18 g
c. volume of O2 : 9.52 L
Further explanationGiven
Reaction
4 NH₃ (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (l)
Required
a. volume of NO
b. mass of H2O
c. volume of O2
Solution
Assume reactants at STP(0 C, 1 atm)
Products at 1000 C (1273 K)and 1 atm
a. mol ratio NO : O2 from equation : 4 : 5, so mo NO :
\(\tt \dfrac{4}{5}\times 0.5=0.4\)
volume NO at 1273 K and 1 atm
\(\tt V=\dfrac{nRT}{P}=\dfrac{0.4\times 0.08206\times 1273}{1}=41.785~L\)
b. 15 L NH3 at STP ( 1mol = 22.4 L)
\(\tt \dfrac{15}{22.4}=0.67~mol\)
mol ratio NH3 : H2O from equation : 4 : 6, so mol H2O :
\(\tt \dfrac{6}{4}\times 0.67=1\)
mass H2O(MW = 18 g/mol) :
\(\tt mass=mol\times MW=1\times 18=18~g\)
c. mol NO at 1273 K and 1 atm :
\(\tt n=\dfrac{PV}{RT}=\dfrac{1\times 35.5}{0.08206\times 1273}=0.34\)
mol ratio of NO : O2 = 4 : 5, so mol O2 :
\(\tt \dfrac{5}{4}\times 0.34=0.425\)
Volume O2 at STP :
\(\tt 0.425\times 22.4=9.52~L\)
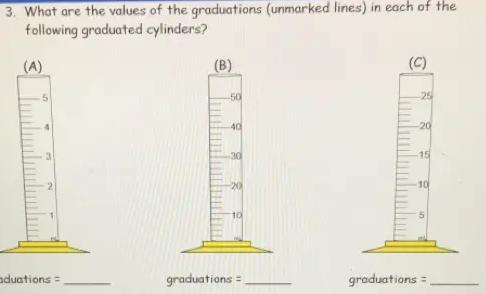
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Why is Hydrogen on Alkali Metal side?
Answers
Hydrogen is an Alkali Metal side because it has ns1 electron configuration like the alkali metals. elements are very reactive
What separates the inner planets from the outer planets in our solar system?
()Comet Belt
()Asteroid Belt
()Their differences
()Distance
Help plss!!
Answers
Answer:
the answer is B Astroid Belt
Ice floats due to its?
Answers
Answer: it floats because it’s less dense than water
Explanation: since it’s less dense it floats
the breakdown of glucose is linked to cellular work by a chemical driveshaft known as
Answers
Answer:
ATP
Explanation:

Which of the following does NOT show a chemical change of matter?
A. rust forming on a metal swingcooking an egg
B. cooking an egg
C. ice cracking
D. burning a piece of firewood
Answers
Answer:
C. Ice cracking
Explanation:
The composition of the ice is still the same therefore, it is an example of a physical change.
when a 12.7 g chunk of zinc dissolves in 500. ml of 1.450 m hcl, what is the concentration of hydrogen ions remaining in the final solution? (asume the volume does not change during the reaction)
Answers
Concentration of hydrogen ions remaining in the final solution is \(.673 M\)
In original solution we have 1.450M of HCl
1.450 M means 1.450 moles of HCl in 1 liter of water
Therefore, number of moles of HCl in 500ml of water will be
= \(1.450*0.5\) moles
= 0.725 moles of HCl
Reaction between HCl and Zn happens when zinc is added -
\(Zn(s) + 2HCl\) → \(ZnCl_{2} + H_{2}\)
Therefore two moles of HCl is needed for 1 mole of zinc
Thus number of moles of HCl needed to react with 12.7g of Zinc will be
= \((12.7/ 65.38 moles of Zn )* (2moles of HCl/mole of Zn)\)
= .388 Moles
So moles of HCl left = \(0.725 - .3888\)
= 0.3365 moles
In 1 Mole of HCl there is 1 mole of H because
\(HCl\) → \(H^{+} + Cl^{-}\)
Therefore in 0.3365 mole HCl there will be 0.3365 moles of H
Thus final concentration of H ions in final solution is =
= \(0.3365mole/0.5 L\)
= 0.673M
To know more about concentration in solution
https://brainly.com/question/22837979
#SPJ4
Five milliliters (mL) of alcohol is poured into a beaker that contains 65 mL of 2% saltwater. Which are the solutes in the new solution?
salt only
alcohol only
salt and water
alcohol and salt
Answers
The solutes in the new solution are alcohol and salt. Therefore, option D is correct.
What is a solution?A solution is a homogeneous mixture composed of two or more substances. A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent, and the solvent is the substance that dissolves the solute.
A solution is considered homogeneous because the solute particles are evenly distributed throughout the solvent. This means that the composition of the solution is the same throughout, and there are no visible differences or separations of the components.
Solutions can be in different states of matter, such as a gas in a gas, a gas in a liquid, a liquid in a liquid, or a solid in a liquid. Therefore, option D is correct.
Learn more about the solution, here:
https://brainly.com/question/30665317
#SPJ5
21: Consider 100 m 3 of atmospheric air, which is an air-water vapor mixture at 100kPa,15 ∘ C, and 40% relative humidity. Find the mass of water and the humidity ratio. What is the dew point temperature of the mixture?
Answers
The mass of water , humidity ratio and dew point temperature in mixture is approximately 1.70 kg , 0.042 and 7 °C respectively.
For finding the mass of water in the air-water vapor mixture, we can use the concept of relative humidity. Relative humidity is the ratio of the partial pressure of water vapor to the saturation pressure of water vapor at the given temperature.
Step 1: Calculate the saturation pressure of water vapor at 15 °C.
Using a psychrometric chart or table, we find that the saturation pressure of water vapor at 15 °C is approximately 1.705 kPa.
Step 2: Calculate the partial pressure of water vapor in the mixture.
The relative humidity of 40% means that the partial pressure of water vapor is 40% of the saturation pressure.
Partial pressure of water vapor = 40% × saturation pressure of water vapor
Partial pressure of water vapor = 0.40 × 1.705 kPa
Partial pressure of water vapor = 0.682 kPa
Step 3: Calculate the mass of water.
To calculate the mass of water, we need to know the specific volume of the air-water vapor mixture.
Specific volume = Total volume / Total mass
Specific volume = 100 m³ / Total mass
The total mass is the sum of the mass of dry air and the mass of water vapor.
Total mass = mass of dry air + mass of water vapor
The mass of dry air can be calculated using the ideal gas law.
PV = mRT, where P is the pressure, V is the volume, m is the mass, R is the gas constant, and T is the temperature.
Rearranging the equation, we have m = PV / RT.
Using the given values:
m (mass of dry air) = (100 kPa) × (100 m³) / (8.314 J/mol·K × 288.15 K)
m (mass of dry air) ≈ 40.16 kg
Now, we can calculate the mass of water vapor:
Mass of water vapor = Partial pressure of water vapor × specific volume
Mass of water vapor = 0.682 kPa × (100 m³ / 40.16 kg)
Mass of water vapor ≈ 1.70 kg
Therefore, the mass of water in the air-water vapor mixture is approximately 1.70 kg.
Next, let's find the humidity ratio, which represents the mass of water vapor per unit mass of dry air.
Humidity ratio = Mass of water vapor / Mass of dry air
Humidity ratio = 1.70 kg / 40.16 kg
Humidity ratio ≈ 0.042
The humidity ratio of the air-water vapor mixture is approximately 0.042.
Finally, let's calculate the dew point temperature, which is the temperature at which the air-water vapor mixture becomes saturated and water vapor begins to condense.
To find the dew point temperature, we need to know the saturation pressure of water vapor at the dew point temperature. At the dew point, the saturation pressure equals the partial pressure of water vapor.
Using the given partial pressure of water vapor of 0.682 kPa, we can refer to a psychrometric chart or table to find that the dew point temperature is approximately 7 °C.
Therefore, the dew point temperature of the air-water vapor mixture is approximately 7 °C.
In summary:
- The mass of water in the air-water vapor mixture is approximately 1.70 kg.
- The humidity ratio of the mixture is approximately 0.042.
- The dew point temperature of the mixture is approximately 7 °C.
To know more about specific humidity refer here:
https://brainly.com/question/33826631?#
#SPJ11