compound 2: 3,5-dibromo-4-hydroxybenzenesulfonic acid how you would synthesize each compound from benzene, toluene, or phenol using the following reactions:
Answers
In all three syntheses, acid-catalyzed reactions were used to introduce functional groups onto the aromatic ring, and oxidation and bromination reactions were used to convert the intermediate compounds into the final product.
To synthesize 3,5-dibromo-4-hydroxybenzenesulfonic acid from benzene, the first step would be to brominate the benzene to form 3-bromophenol using a mixture of sulfuric acid and bromine. Next, the 3-bromophenol would undergo a reaction with concentrated sulfuric acid to form 3-bromophenolsulfonic acid. This compound would then be oxidized using a strong oxidizing agent like potassium permanganate to form 3-bromo-4-hydroxybenzenesulfonic acid. Finally, this compound would undergo a second bromination step to form 3,5-dibromo-4-hydroxybenzenesulfonic acid.
To synthesize 3,5-dibromo-4-hydroxybenzenesulfonic acid from toluene, the first step would be to oxidize the toluene using potassium permanganate to form benzoic acid. Next, the benzoic acid would undergo a reaction with phosphorus pentabromide to form 3,5-dibromobenzoic acid. This compound would then undergo a reaction with sulfuric acid to form 3,5-dibromobenzenesulfonic acid. Finally, this compound would be hydrolyzed using sodium hydroxide to form 3,5-dibromo-4-hydroxybenzenesulfonic acid.
To synthesize 3,5-dibromo-4-hydroxybenzenesulfonic acid from phenol, the first step would be to brominate the phenol using a mixture of sulfuric acid and bromine to form 2,4,6-tribromophenol. Next, the 2,4,6-tribromophenol would undergo a reaction with concentrated sulfuric acid to form 2,4,6-tribromophenolsulfonic acid. This compound would then undergo a hydrolysis reaction using sodium hydroxide to form 3,5-dibromo-4-hydroxybenzenesulfonic acid.
To know more about acid-catalyzed reactions Visit:
https://brainly.com/question/23970995
#SPJ11
Related Questions
compare the magnitude of the lattice energy for each of the following two ion pairs. (a) cs and br- separated by a distance of 441 pm (b) mn2 and o2- separated by a distance of 292 pm
Answers
E= -5.23 × 10^-19 J/ion pair for Cs+ Br- ion pair.
E= -1.58 × 10^-18 J/ion pair for Mn2+ O2- ion pair.
therefore, lattice energy of second compound is higher than the first one.
Lattice energy is the bond energy of the compound which is now seperated.
Formula: E = (kq1q2)/r
now inserting the values for Cs+ and Br- into the equation,
where q1 = +1, q2 = −1, and r = 441 pm
Energy associated with the formation of a single pair of Cs+Br-
E= 2.31×10^-28 (+1)(-1)/441 × 10^-12
E= -0.00523 × 10^-16
E= -5.23 × 10^-19 J/ion pair
now inserting the values for Mn+2 and O2- into the equation,
where q1 = +2, q2 = −2, and r = 292 pm
Energy associated with the formation of a single pair of Mn+2O-2
E= 2.31×10^-28 (+2)(-2)/292× 10^-12
E= -0.0158 × 10^-16
E= -1.58 × 10^-18 J/ion pair
Therefore comparing both the ion pairs , the lattice energy of Mn+2 O2- pair is higher than the Cs+ Br- ion pair.
To learn more about lattice energy visit the link- https://brainly.com/question/13169815
#SPJ4
Use this oxidation-reduction reaction to answer questions about half-reactions:
Upper m g plus 2 upper H upper C l right arrow upper M g upper C l subscript 2 plus upper H subscript 2.
Classify these half-reactions by typing in “O” for “oxidation half-reaction,” “R” for “reduction half- reaction,” and “N” for “neither.”
Answers
Answer:
For those who need it, the answers are N, O, R
Explanation:
To solve such this we must know the concept of redox reaction. Therefore, the given reaction is an oxidation and reduction reaction. The oxidation reaction is Mg \(\rightarrow\) MgCl\(_2\) and reduction reaction is HCl \(\rightarrow\) H\(_2\) .
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Redox reaction is a chemical reaction where oxidation and reduction takes place simultaneously. Oxidation is loss of electrons and reduction is gain of electrons. The electron transfer from oxidant to reductant.
Mg + 2HCl \(\rightarrow\) MgCl\(_2\) + H\(_2\)
Mg \(\rightarrow\) MgCl\(_2\) O
HCl \(\rightarrow\) H\(_2\) R
Therefore, the given reaction is an oxidation and reduction reaction.
Learn more about the chemical reactions, here:
brainly.com/question/3461108
#SPJ2
Pls help me will give brainliest.
Where is convection happening on Earth?
A. Atmosphere
B. Earth's outer core
C. Earth's mantle
D. Oceans
E. All of the above I
Answers
Answer:
C. Earth's Mantle
Explanation:
Answer:
C
Explanation:
Earth's mantle is the correct answer
Compound A solid
Compound B solid
Compound
C
Compound
D
B
C
gas
D
solid
808
1610
-56
680
yes
no
no
Compound B because it has a very high melting point.
yes
You are studying the differences between ionic and covalent compounds. After studying the properties of each
type of compound, your teacher has provided this table and asked you to (1) identify the ionic compound(s) and
explain your choice(s).
A
Compounds A and D because they have relatively high melting points.
AROLIChessies
Compounds A, B, and C because they are solid at room temperature.
Compounds A and D because the conduct electricity in aqueous solutions.
yes
no
yes
yes

Answers
The Compounds A, B, and C are ionic compounds because they are solid at room temperature. Therefore, option B is correct.
What are ionic compound ?A chemical compound known as an ionic compound is one that contains ions bound together by the electrostatic forces known as ionic bonding. Despite having both positively and negatively charged ions, or cations and anions, the molecule is generally neutral.
Ionic chemicals are brittle and rigid. When an ionic chemical is dissolved in water, it separates into ions. Ionic compound solutions and melting forms of these substances carry electricity, while solid materials do not. The chemical formula of an ionic compound is metal + nonmetal or polyatomic ions.
Thus, option B is correct.
To learn more about an ionic compound, follow the link;
https://brainly.com/question/9167977
#SPJ1
what are the 4 quantum numbers for the last electron in promethium 
Answers
Answer:
Promethium atoms have 61 electrons and the electronic shell structure is [2, 8, 18, 23, 8, 2] with Atomic Term Symbol (Quantum Numbers) 6H5/2.
What is the Index Value for an exposure of low?
Answers
The concept is to provide diagnostic images using the optimal amount of radiation. If the index is too low, it may indicate poor image quality. If the dose index is too high, it may mean using more radiation than is needed for imaging.
Various Kinds of Index NumbersThere are three kinds of index numbers, namely:
a. Price index figures, namely comparative figures to measure price changes from one period to another.
b. Number index (quantity), which is a comparison number to measure changes in the amount from one period to another.
c. Value index number, which is a comparison number to measure changes in value from one period to another. Value is calculated by multiplying the price by the amount (quantity).
Your question is incomplete but most probably your full question was:
Explain. What is the Index Value for an exposure of low?
Learn more about index value at https://brainly.com/question/8154168.
#SPJ1
Which word goes we’re ?

Answers
2.arm
3. Diaphram
4. Base
5. Light source
6. Tube
7. Stage clips
8. Objectives
9. revolving nose piece
10. eye piece
11. Stage
T/F
The transportation term of sale known as Free on Board (FOB) origin requires that the seller legally retains ownership of the product from the point of origin until it reaches the buyers destination
Answers
True. Free on Board (FOB) is a term of sale that establishes who is responsible for the costs of shipping and receiving the goods.
The seller is legally responsible for the goods until they are loaded onto the shipping vessel and the buyer is responsible for the shipment from then on. This helps to ensure that the buyer and seller both understand who is responsible for what costs associated with the shipment.
The Role of Free on Board (FOB) in International TradeThe Free on Board (FOB) term of sale is an essential component of many international trade agreements. FOB is a legal term that establishes who is responsible for the costs of shipping and receiving goods. It is used to ensure that the buyer and seller both understand who is responsible for what costs associated with the shipment. This helps to ensure that both parties are protected in the case of any issues that may arise during the shipping process.
The FOB term of sale is especially beneficial for international trade. In the case of international trade, the seller is legally responsible for the goods until they are loaded onto the shipping vessel. This means that the seller must ensure that the goods are in good condition and fit for shipping. This helps to protect the buyer's interests and ensures that the goods are delivered in a timely and satisfactory manner.
Learn more about Free on Board (FOB) :
https://brainly.com/question/16000629
#SPJ4
Two children on a seesaw are able to balance perfectly while on Earth. Would they still be balanced if the seesaw was brought to the moon?(1 point)
No, they would not be balanced because their weights would change.
No, they would not be balanced because their masses would change.
Yes, they would still be balanced because their weights would not change.
Yes, they would still be balanced because their masses would not change.
Answers
Answer:
No, they would not be balanced because their weights would change.
Explanation:
Weight is the effect that gravitational pull has on objects on Earth whereas mass is how much matter there is in an object. Mass does not change despite gravitational pull, but the force of gravity (weight) is different depending on where you are.
please help when ever it lets me give brainliest i will give it
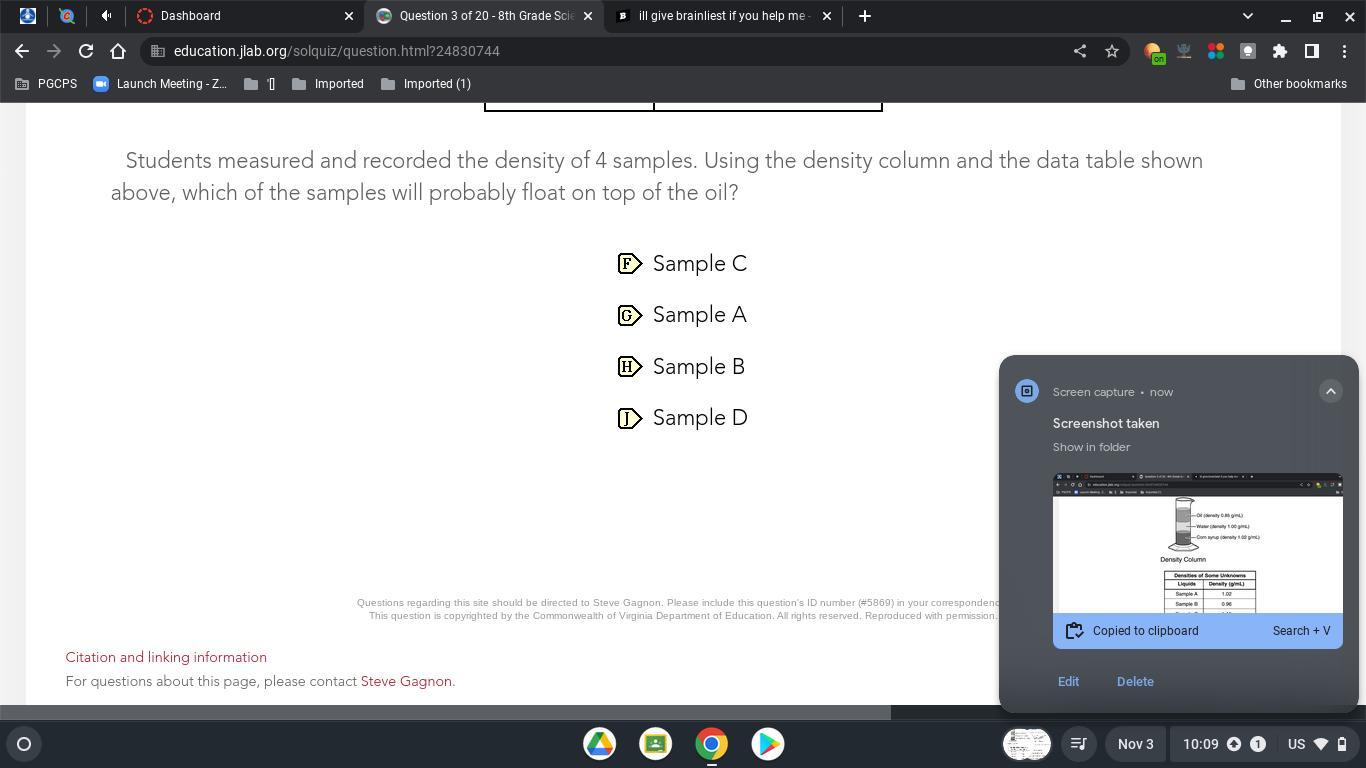

Answers
Answer:
it's sample c yh it is true
explain why no precepitate formed when solutions of iron)iii) chloride and sodium sulfate were combined
Answers
No precepitate formed because all salt formed in the reaction are soluble in water.
Balanced chemical reaction: 2FeCl₃(aq) + 3Na₂SO₄(aq) → Fe₂(SO₄)₃(aq) + 6NaCl(aq)
This chemical reaction is double displacement reaction - cations and anions of the two reactants switch places and form two new compounds.
FeCl₃ is iron(III) chloride and it is soluble in water
Na₂SO₄ is sodium sulfate and it is soluble in water
Fe₂(SO₄)₃ is iron(III) sulfate and it is soluble in water
NaCl is sodium chloride and it is soluble in water
aq means soluble in water
In this chemical reaction, there are no acids and bases, only the salts.
More info about reactions: brainly.com/question/17127386
#SPJ4
List all possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom.
Answers
In quantum mechanics, the angular momentum quantum number "l" defines the shape of the atomic orbital. The l value is an integer ranging from 0 to (n-1) where n is the principal quantum number.
Therefore, for an electron in the L(n=2) shell of an atom, the possible values of the angular momentum quantum number l would range from 0 to 1, since n=2.
This is because the L shell is the second shell, which has n=2. Therefore, it can have subshells with l=0 and l=1, also known as the s and p subshells respectively.
The angular momentum quantum number also has an effect on the energy of the electron, with higher l values having higher energy.
Thus, the possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom are l=0 and l=1.
To know more about quantum mechanics refer here: https://brainly.com/question/23780112#
#SPJ11
Pls help!!! i'll mark you the brainlest
the data point for question 16 should be thrown out because it is a (n)
A.distance
B.outliner
C.wrong surface type
D.fundemental unti
Answers
A water has a pH of 8.0 and the concentration of HCO3 is 1.5 x 10-3 M. What is the approximate alkalinity of the water in units of mg/L as CaCO3?
Answers
The approximate alkalinity of the water in units of mg/L as CaCO3 using the equation.
To determine the approximate alkalinity of the water in units of mg/L as CaCO3, we need to calculate the concentration of bicarbonate ions (HCO3-) and convert it to units of CaCO3.
The molar mass of CaCO3 is 100.09 g/mol, and we can use this information to convert the concentration of HCO3- to mg/L as CaCO3.
First, let's calculate the alkalinity:
Alkalinity = [HCO3-] * (61.016 mg/L as CaCO3)/(1 mg/L as HCO3-)
Given:
pH = 8.0
[HCO3-] = 1.5 x 10^(-3) M
Since the pH is 8.0, we can assume that the water is in equilibrium with the bicarbonate-carbonate buffer system. In this system, the concentration of carbonate ions (CO3^2-) can be calculated using the following equation:
[CO3^2-] = [HCO3-] / (10^(pK2-pH) + 1)
The pK2 value for the bicarbonate-carbonate buffer system is approximately 10.33.
Let's calculate the concentration of CO3^2-:
[CO3^2-] = [HCO3-] / (10^(10.33 - 8.0) + 1)
= [HCO3-] / (10^2.33 + 1)
= [HCO3-] / 234.7
Substituting the given value:
[CO3^2-] = (1.5 x 10^(-3) M) / 234.7
Now, we can calculate the alkalinity:
Alkalinity = [HCO3-] + 2 * [CO3^2-]
= (1.5 x 10^(-3) M) + 2 * (1.5 x 10^(-3) M) / 234.7
= (1.5 x 10^(-3) M) + (3 x 10^(-3) M) / 234.7
To convert alkalinity to mg/L as CaCO3, we use the conversion factor:
1 M = 1000 g/L
1 g = 1000 mg
Alkalinity (mg/L as CaCO3) = Alkalinity (M) * (1000 g/L) * (1000 mg/g) * (100.09 g/mol)
= Alkalinity (M) * 100,090 mg/mol
Substituting the calculated value:
Alkalinity (mg/L as CaCO3) = [(1.5 x 10^(-3) M) + (3 x 10^(-3) M) / 234.7] * 100,090 mg/mol
Now, you can calculate the approximate alkalinity of the water in units of mg/L as CaCO3 using the above equation.
To learn more about equation visit;
https://brainly.com/question/29657983
#SPJ11
The pressure of a gas is 780 torr when its volume is 400. mL and the temperature is 20.0°C. If the gas is allowed to expand to 500. mL at 25.0°C, what will the pressure be?
Answers
As volume increases, pressure decreases. This can be deduced from the equation above: P1V1 equals P2V2 and so on. P2 = (780 torr)(400 mL) (500 mL) P2 = 780 torr x 400/500 mL/ 624 torr pressure.
What will the gas's pressure be?As a result, the gas pressure that a gas experiences is equal to the product of the forces the gas molecules exert on the walls of the its container divided by the entire surface area of a container walls.
Why is there pressure in petrol?The force created when gas molecules slam into surfaces of objects produces gas pressure (Figure 1). Even though there is very little force involved in each collision, any surface with a sizeable area is subject to many of them quickly, which can lead to a high pressure.
To know more about deduced visit:
https://brainly.com/question/20711322
#SPJ1
Write any three possible structures for octane, C8H18 and give their IUPAC names
Answers
Answer:
Octane (n- octane) C8H184 - Methylheptane3- Ethylhexane2 ,2 - Dimethylhexanehope it helps

The three possible structures for octane, C8H18 and give their IUPAC names are -
Octane2-Methylheptane2,2-Dimethylhexane(structures are attached)
The octane is a hydrocarbon with 8 carbon atoms and 18 hydrogen atoms. The molecular formula of octane is C8H18.
Carbon is a tetravalent atom that contains 4 electrons in the valence shell.carbon can donate its four electrons to form bonds with four other atoms.The structure of octane is drawn by first drawing a straight-chain or parent chain of 8 carbons and attaching 18 hydrogens on carbon to fulfill its tetravalency.The three possible structures for octane, C8H18 and give their IUPAC names are -
Octane2-Methylheptane2,2-DimethylhexaneLearn more about:
https://brainly.com/question/1370895

what is a food web made up of
Answers
Answer:
A food web is made up of all the food chains in a single ecosystem. Each living thing in an ecosystem belongs to many food chains. A food chain is a path that energy takes through a certain ecosystem. Trophic Levels. Organisms in food webs are grouped into categories called trophic levels.
Explanation:
I HOPE THIS HEPLS HAVE A WONDERFUL DAY!:)
Answer:
you didnt say if it was an animal or people food web so imma just try my best to explane tis is an animal food web. So it really is just showing was the animals eat and if they eat other animal basicly.
Explanation:
7. At high concentrations in aqueous solutions, palmitic acid assembles into liposomes, which are structurally equivalent to membrane lipid bilayers. Given this information, which of the following statements best describes the structure of the liposome? A. The liposome contains two layers of palmitic acid, where the methyl ends of the molecules point towards solvent and the carboxylic acid ends point towards each other. B. The liposome contains two layers of palmitic acid, where the methyl ends of the molecules point towards each other and the carboxylic acid ends point towards solvent Both the methyl and carboxylic acid ends of the palmitic acid molecules are exposed to solvent. The liposome has no C. D. 8. According to Figures 2-7 & 2-8 in the textbook, the aqueous solvation of which of these metabolites would result in the greatest loss in entropy? A. Glucose B. Palmitic acid C. Vitamin K1
Answers
7. The correct statement describing the structure of the liposomes is option A. The liposome contains two layers of palmitic acid, where the methyl ends of the molecules point towards the solvent and the carboxylic acid ends point towards each other.
Liposomes are formed when amphiphilic molecules, such as palmitic acid, arrange themselves in a bilayer structure in aqueous solutions. The hydrophobic (methyl) ends of the molecules tend to interact with each other and face inward, while the hydrophilic (carboxylic acid) ends face the solvent. This structure allows liposomes to encapsulate substances within their internal space, creating a protected environment. It mimics the structure of cell membranes, making liposomes useful for drug delivery systems and other applications in biotechnology. A liposome is a small spherical vesicle composed of lipid molecules, commonly used in drug delivery and biotechnology applications.
Learn more about liposomes here:
https://brainly.com/question/32221162
#SPJ11
Which of the following functional groups CANNOT hydrogen bond with itself? (select all) 1) Ethers 2) Tertiary amines 3) Esters 4) Carboxylic acids
Answers
Among the given options, the functional group that cannot hydrogen bond with itself is: 1) Ethers Ethers, which have the general formula R-O-R', consist of two alkyl or aryl groups bonded to an oxygen atom.
While oxygen is capable of forming hydrogen bonds with hydrogen atoms from other functional groups, ethers themselves do not have hydrogen atoms directly bonded to the oxygen atom. As a result, ethers lack the necessary hydrogen bonding donor or acceptor sites required for intermolecular hydrogen bonding.
2) Tertiary amines: Although they lack a hydrogen atom directly bonded to the nitrogen atom, they can still participate in hydrogen bonding as hydrogen bond acceptors.
3) Esters: The oxygen atom in the ester functional group can act as both a hydrogen bond donor and acceptor, enabling intermolecular hydrogen bonding.
4) Carboxylic acids: Carboxylic acids have a hydrogen atom bonded to the oxygen of the carboxyl group, making them capable of forming hydrogen bonds with other carboxylic acid molecules through the oxygen and hydrogen atoms.
Learn more about functional group here: brainly.com/question/31328777
#SPJ11
How would you decarboxylate this compound, r-ch2ch2cooh? (there are multiple ways.)
Answers
To decarboxylate the compound R-CH2CH2COOH (where R represents any organic group), there are several methods that can be employed.
One common method involves heating the compound at high temperatures. This can be achieved by using an oven or heating mantle to apply heat in the range of 150-200 degrees Celsius.
Another way is to use a catalyst such as sodium or potassium hydroxide, which helps in the decarboxylation process. In this method, the compound is mixed with the catalyst and heated to initiate the decarboxylation reaction.
Alternatively, decarboxylation can also be accomplished through biological processes using enzymes. For example, certain bacteria and fungi produce enzymes that facilitate decarboxylation reactions.
Overall, the decarboxylation of R-CH2CH2COOH can be achieved through heating, using catalysts, or biological methods, depending on the specific requirements of the reaction.
To know more about decarboxylate visit:
https://brainly.com/question/33440364
#SPJ11
Explain why, in a balanced chemical equation C + O2 CO2, we know that 1 gram of C will not react exactly with 1 gram of O2.
Answers
Answer:
See the answer below
Explanation:
From the equation, 1 mole of C requires 1 mole of O2 for a balanced reaction.
1 g of C will not exactly react with 1 g of O2 because the 1 g of each of the reactants contain different amounts of the substance.
The amount (in moles) of a substance is the mass of that substance divided by the molar mass.
Hence, 1 g of C will contain 1/12 moles of the substance while 1 g of O2 will contain 1/32 moles of the substance.
what is global warming
Answers
how much volume do the gas molecules take up in an inflated balloon
Answers
Answer:
The volume of a gas is determined by the volume of the container it is in. Gases take the shape of their container and the volume of the container....
Explanation:
What happens to the volume or size of a balloon as it rises in the air?
When a balloon goes up higher in the air, its size will increase. Since there's less air in the upper atmosphere, there's less stuff pushing back on the balloon, and hence the pressure is lower, which allows the balloon to expand.
Is pressure inside a balloon greater?
The pressure inside the balloon is higher than the pressure on the outside. ... And the direction of airflow is always from higher to lower pressure. If the pressure was same then there would be no airflow and if the pressure outside was higher , air will flow from outside to the inside of the balloon.
What is the relationship between volume and pressure?
Boyle's law is a gas law, stating that the pressure and volume of a gas have an inverse relationship. If volume increases, then pressure decreases and vice versa, when temperature is held constant. Therefore, when the volume is halved, the pressure is doubled; and if the volume is doubled, the pressure is halved.
Mark as brainliest if liked it. Thanks hope this helps
Which organelle of a prokaryotic cell contains genetic information?
cell membrane
ribosomes
nucleus
nucleoid
Answers
Answer:
The right option is the 4 one. nucleoid
Prokaryotic cells are simple cells that lack a definite nucleus and some membrane-bound organelles. Prokaryotic cells have a nucleoid region, which is an irregularly-shaped central region of the cell that contains the cell’s genetic information (DNA). Other organelles that can be found in prokaryotic cells include plasma membrane, cell wall, cytoplasm, and ribosomes.
Explanation:
Answer:
nucleoid
Explanation:
Which of these is true about pure substances? They can only contain one type of molecule. They may contain one type of atom or one type of molecule. They can only contain one type of atom. They can contain different types of atoms and molecules.
Answers
Answer:
its totally d
Explanation:
*extra points*
Balancing chemical equations - I'm having some trouble with this question, can someone please help me out?

Answers
Answer:
3h2so4+2b(oh)3=b2(so4)3+6h2o
Wave Rock is a granite outcropping in western Australia.
The curved cliff has been weathered by water, which
changed some of the minerals that make up the granite
and caused the streaks of color.
Which type of weathering caused the streaks of color?
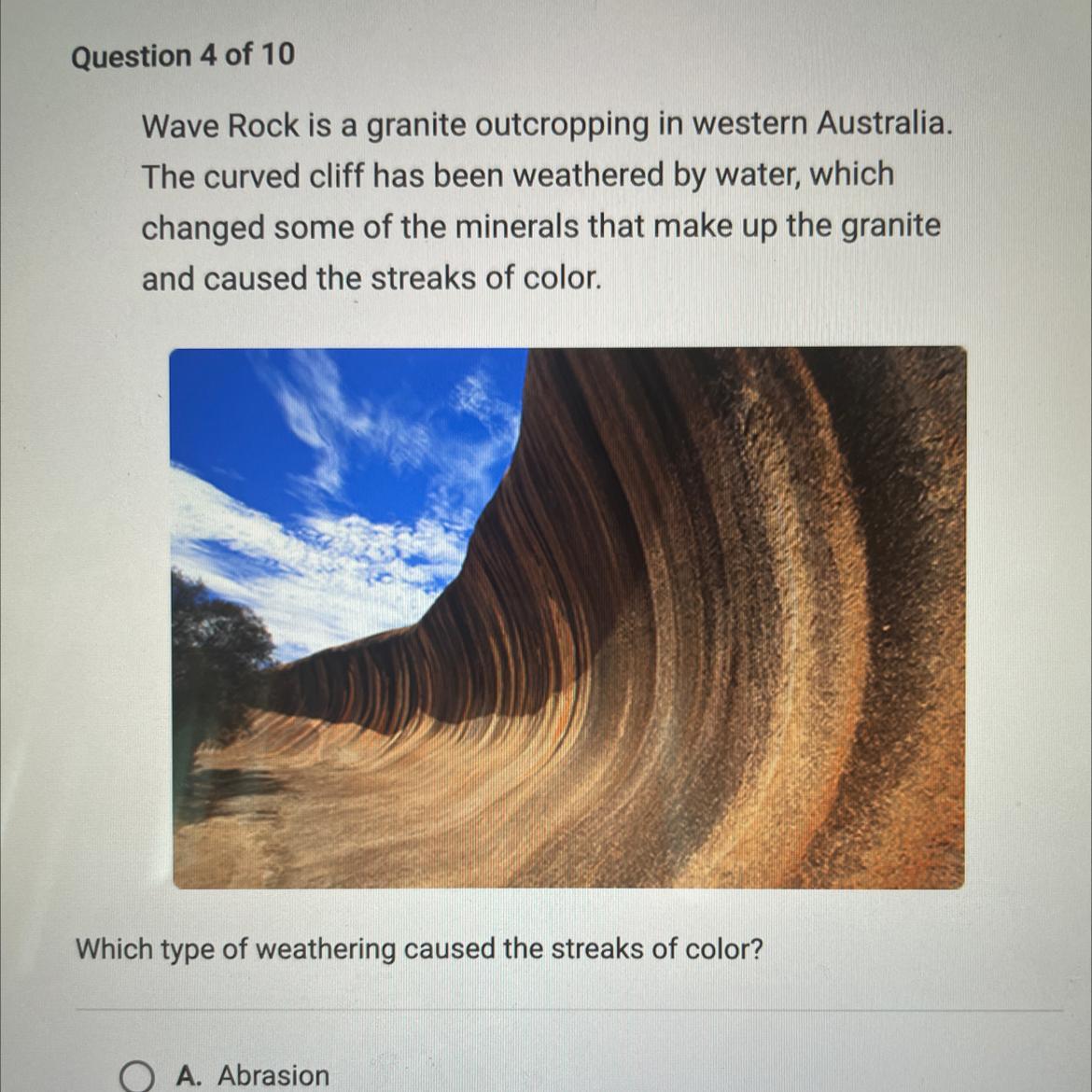
Answers
The type of weathering caused by the streaks of color is chemical weathering.
What is chemical weathering of the rock about?Chemical weathering occurs when the minerals in a rock are chemically altered by exposure to water, acids, or other chemicals. This process can change the composition of the rock and cause it to take on new colors or patterns.
In the case of Wave Rock, it is likely that the streaks of color were caused by the alteration of minerals in the granite by water. Water can dissolve minerals in rocks and cause them to leach out, leaving behind new minerals that may have different colors. This process can result in the formation of streaks or patterns on the surface of the rock.
Note that Physical weathering, on the other hand, occurs when the physical structure of a rock is changed by mechanical forces such as freezing and thawing, abrasion, or pressure. This type of weathering does not typically result in changes to the chemical composition of the rock, and is not likely to be the cause of the streaks of color on Wave Rock.
Learn more about chemical weathering from
https://brainly.com/question/29784758
#SPJ1
If the centre of an atom contains 8 particle that are charged, how many particles are revolving round this centre?
Answers
Explanation:
charged particles=8 which is proton and proton=no.of electron. That's why 8 particles are revolving round this center. And this atom structure is of oxygen
If the center of an atom has 8 charged particles that are protons as the neutrons are neutral there will be 8 negative charges that are electrons revolving around the center nucleus.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether it is solid,liquid or gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged particles and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged particles and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
Perform the following operationand express the answer inscientific notation.8.6500x103 + 6.5500x105[? ]x10!?)

Answers
First, we need to make the exponent of 10 the same for both.
So let's transform 6.5500 x 10^5 into some number x 10^3.
For this, we need to move the dot to the right, some places where it gives the number 3. In this case, 2 places.
655.00 x 10^3
now we can sum the numbers
8.6500 x 10^3 + 655.00 x 10^3 = 663.65 x 10^3
now we need to transform this number into scientific notation. For this, must have only one number before the dot(on the left side of the dot). We will move the dot to the left, 2 places:
6.6365 x 10^5
Answer: 6.6365 x 10^5
Equimolar amounts of Cl2(g) and CO(g) are injected into an evacuated, rigid container, where they react according to the equation below. Cl2(g)+CO(g)⇄COCl2(g) ΔHrxn=−109kJ/molrxn (a) If 7.0 g of CO(g) is consumed in the reaction with excess Cl2(g), how many moles of COCl2(g) are produced? (b) Which element is oxidized in this reaction? Justify your answer in terms of oxidation numbers.
Answers
Answer:
0.25 moles of COCl₂ are been produced
The element that is oxidized is C, it changed the oxidation state from +2 in CO to +4 in phosgene.
Explanation:
Equilibrium reaction:
Cl₂(g) + CO(g) ⇄ COCl₂(g)
Let's convert the mass of CO to moles:
7g . 1mol /28g = 0.25 moles
As ratio is 1:1, we can say that 0.25 moles of COCl₂ are been produced.
1 mol of chlorine reacts to 1 mol of carbon monoxide in order to produce 1 mol of phosgene.
Chlorine is been reduced:
Cl₂ + 2e⁻ ⇄ 2Cl⁻
Change the oxidation state, from 0 (ground state) to -1. Oxidation state decreased.
Carbon is been oxidized.
In CO, carbon has +2 as oxidation state. In phosgene the oxidation state is +4. This oxidation state was increased, that's why it has oxidized.
The element that is oxidized is carbon whose oxidation number was increased from +2 to +4.
The equation of the reaction is;
Cl2(g) + CO(g) ⇄ COCl2(g)
We have been told that the chlorine gas is the reactant in excess hence the carbon monoxide is the limiting reactant.
Number of moles of CO = 7.0 g/28 g/mol = 0.25 moles
Since the reaction is 1:1, 0.25 moles of COCl2 is produced.
The element that is oxidized is carbon whose oxidation number was increased from +2 to +4.
Learn more about oxidation: https://brainly.com/question/4260635