Determine the correct characteristics to recognize a covalent compound.
Answers
Covalent bonds are formed by sharing electrons. Covalent compounds are also known as molecular compounds, and they typically have low melting and boiling points. These are some characteristics that can help identify covalent compounds:Electron Sharing: Covalent compounds are formed when two or more atoms share valence electrons with one another.
Atoms with similar electronegativity will tend to share electrons, which leads to the formation of covalent bonds. Covalent bonds can be polar or nonpolar, depending on the difference in electronegativity between the two atoms involved in the bond.Low Melting and Boiling Points: Covalent compounds generally have lower melting and boiling points than ionic compounds. This is because covalent compounds are held together by weak intermolecular forces rather than strong electrostatic forces. This makes them easier to melt or boil.Molecular Shape: Covalent compounds are typically made up of discrete molecules that are held together by covalent bonds. The shape of these molecules is determined by the arrangement of their atoms and the number of lone pairs of electrons around the central atom.Electrical Conductivity: Covalent compounds do not conduct electricity in the solid or liquid state, but they can conduct electricity when dissolved in water or other polar solvents. This is because the water molecules can break apart the covalent bonds and create ions that are able to carry an electric charge.
For more information on Covalent bonds visit:
brainly.com/question/19382448
#SPJ11
Related Questions
Hi hello I need help ASAP
Determine the slope of this line
a.2.5
b.0.75
c.1
d.0.4

Answers
Answer:
so I got it wrong the first time but you should pick two points on the line such as 4,3 and 9,5. Then use the equation y^2 - y^1/ x^2 - X^1. You would get 2/5 which is D.
Explanation
What are the cons of using a cup made of steel?
Answers
Answer:
1. Sometimes there’s a metallic taste to the water
2. The water becomes hot if left in your car or outdoors in hot weather
3. Bottle can dent if dropped
4. Paint sometimes peels off exterior of metal bottles
5. Metal water bottles lined with a resin lining also leach BPA. Do not get a bottle with what appears to be a “colored” lining of any kind.
Explanation:
Answer:
Steel feels cold, can dent, and is heavy. Sometimes it has a strange taste. Steel can also stain with use.
Explanation:
Hope this helps! :)
To be effective, a certain drug must not drop below 15 mg in the body. A 120
mgs of that drug was administered to a patient. How much time will it take for that drug to reach the 15 mg minimum level. The drug's half-life is 30 minutes.(show how you arrived at your response)
Answers
Answer:
90 Minutes
Explanation:
Original concentration = 120 mg
Half life = 30 minutes
Minimum level = 15 mg
Basically, we are to calculate the time it would take to drop from 120 mg to 15 mg.
The half life of a substance is the time it takes for the original concentration of a substance to drop to half it's starting concentration.
First Half life:
Starting concentration = 120mg
Concentration after half life = 120 / 2 = 60 mg
Second Half life;
Starting concentration = 60 mg
Concentration after half life = 60 / 2 = 30 mg
Third Half life;
Starting concentration = 30 mg
Concentration after half life = 30 / 2 = 15 mg
We have reached the minimum 15 mg level. This means it took three half lives to get there.
Total time taken = No of Half lives * Half life duration = 3 * 30 mins = 90 Minutes
A container of gas has a volume of 280 mL at a temperature of 22 Celsius if the pressure remains constant what is the volume 44 Celsius?
Answers
Answer:
300.9mL
Explanation:
Given parameters:
V₁ = 280mL
T₁ = 22°C
T₂ = 44°C
Unknown:
V₂ = ?
Solution:
To solve this problem, we apply Charles's law;
it is mathematically expressed as;
\(\frac{V_{1} }{T_{1} } = \frac{V_{2} }{T_{2} }\)
We need to convert the temperature to kelvin;
T₁ = 22°C = 22 + 273 = 295K
T₂ = 44°C = 44 + 273 = 317K
Input the parameters and solve;
\(\frac{280}{295}\) = \(\frac{V_{2} }{317}\)
V₂ x 295 = 280 x 317
V₂ = 300.9mL
The molarity of a NaOH solution was determined by titration with KHP. The results of five titrations were 0.1025 M, 0.1087 M, 0.1100 M, 0.1052 M, 0.0997 M. Answer the following questions based on 95% confidence level.
a) Calculate the absolute standard deviation of the concentration of NaOH.
b) Calculate the standard error of the concentration of NaOH.
c) Calculate the confidence interval of the concentration of NaOH. Report your answer with appropriate significant figures
d) If the true concentration of this NaOH solution is 0.1045 M, is the sample mean significantly different from the true concentration?
e) Another student also measured the concentration of the same NaOH solution. The result of the three titrations were 0.1028 M, 0.1012 M, 0.0983 M. Are the mean concentrations from the two students’ result similar to each other?
Answers
a) The absolute standard deviation of the concentration of NaOH is 0.0041 M.
b) The standard error of the concentration of NaOH is 0.0018 M.
c) The confidence interval of the concentration of NaOH is (0.1033 M, 0.1060 M).
d) Yes, the sample mean is significantly different from the true concentration of 0.1045 M.
e) No, the mean concentrations from the two students' results are not similar to each other.
a) To calculate the absolute standard deviation of the concentration of NaOH, we need to find the standard deviation of the given data points. Using the formula for sample standard deviation, we calculate the average deviation of each data point from the mean concentration, then square each deviation, take the average of the squared deviations, and finally, take the square root. The absolute standard deviation is the absolute value of the standard deviation.
b) The standard error of the concentration of NaOH measures the variability of the sample means from different samples. It is calculated by dividing the standard deviation by the square root of the sample size. In this case, the sample size is 5.
c) To calculate the confidence interval of the concentration of NaOH, we need to determine the margin of error using the t-distribution and the sample standard deviation. With a 95% confidence level, we use a t-value corresponding to 4 degrees of freedom (n-1) and multiply it by the standard error. The confidence interval is constructed by subtracting and adding the margin of error to the sample mean concentration.
d) To determine if the sample mean is significantly different from the true concentration, we compare the true concentration to the confidence interval. If the true concentration falls outside the confidence interval, then the sample mean is significantly different from the true concentration.
e) To assess if the mean concentrations from the two students' results are similar to each other, we can calculate the confidence intervals for each student's data. If the confidence intervals overlap or are close to each other, it suggests that the mean concentrations are similar. However, if the confidence intervals do not overlap, it indicates that the mean concentrations are likely different.
Learn more about concentration
brainly.com/question/18247103
#SPJ11
Give an example of :
-Nonpolar covalent compound
-Polar covalent compound
-Ionic compound
Answers
Answer:Nonpolar covalent compound-
Carbon tetrachloride CCl4
Polar covalent compound-Water - H2O.
Ammonia - NH. ...
Sulfur dioxide - SO. ...
Hydrogen sulfide - H2S.
Ionic compoundNaCl, sodium chloride ordinary table salt
Al(OH)3, aluminum hydroxide ingredient in antacids
NaOH, sodium hydroxide lye; used as drain cleaner
K3PO4, potassium phosphate
Explanation:
have a great day ahead" :)
help me with this ?
i need to git this done for school
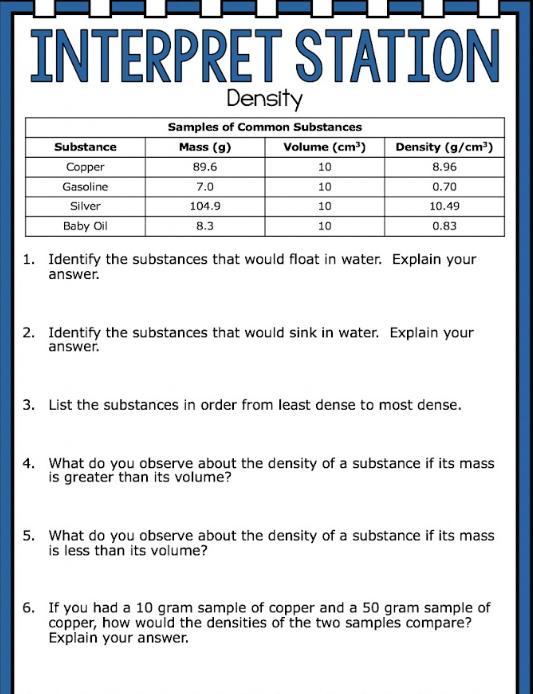
Answers
1) Gasoline and baby oil will float in water
2) Copper and silver will sink in water
3) Silver, Copper, Baby oil Gasoline
4) If the mass is less than volume the density will be less than one
5) If the mass is greater than the volume the density is not less than 1
6) The densities of the substances would be the same.
What is the density?Density is a physical property of matter that measures how much mass is contained in a given volume. It is calculated as the ratio of mass to volume, and is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
The formula for calculating density is:
Density = Mass / Volume
Where Mass is the amount of matter in an object or substance, and Volume is the amount of space that it occupies. Density is an intensive property, which means that it is independent of the amount of substance being measured. For example, the density of a material will be the same regardless of whether you measure it in grams or kilograms.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
When the [CO] and [H2CO3] are both horizontal
lines, the rate of the forward reaction is
the rate of the reverse reaction
a.faster than
b. slower than
C. the same as
Answers
When the [CO] and [H2CO3] are both horizontal lines, the rate of the forward reaction is the same as the rate of the reverse reaction.
The statement indicates that the concentrations of carbon monoxide ([CO]) and carbonic acid ([H2CO3]) remain constant over time, suggesting that the system has reached a state of dynamic equilibrium. In a reversible reaction, equilibrium is achieved when the rate of the forward reaction is equal to the rate of the reverse reaction.
At equilibrium, the concentrations of reactants and products no longer change, but it does not mean that the reactions have stopped. Instead, the forward and reverse reactions continue to occur, but at an equal rate, resulting in a constant concentration of species. If the rates of the forward and reverse reactions were not equal, the system would not be at equilibrium. In such cases, the concentrations of the reactants and products would change over time until equilibrium is established.
Therefore, when the concentrations of [CO] and [H2CO3] are both horizontal lines, it implies that the rates of the forward and reverse reactions are equal, indicating that the system has reached a dynamic equilibrium state.
To learn more about dynamic equilibrium click here:
brainly.com/question/2033603
#SPJ11
Is 4Na2CO3 a mixture why or why not?
Answers
Answer:
In the United States today, no federal law prohibits human cloning, either for purposes of reproduction or for purposes of biomedical research.
Explanation:
hypothetically, if you could make an aqueous solution of both nabr and agf , what is produced at each electrode during electrolysis?
Answers
If you were to make an aqueous solution of both NaBr and AgF and subjected it to electrolysis, different products would be produced at each electrode. At the anode, Br- ions would be oxidized to form Br2(g) gas and electrons. The overall reaction occurring at the anode is 2Br- → Br2(g) + 2e-.
At the cathode, Ag+ ions would be reduced to form solid silver (Ag) and electrons. The overall reaction occurring at the cathode is Ag+ + e- → Ag(s).
It is important to note that during electrolysis, the cations and anions present in the solution are attracted to opposite electrodes due to their opposite charges. This results in a separation of the ions and their subsequent reactions at the electrodes.
Additionally, it is worth noting that the process of electrolysis can be used to selectively deposit metals onto surfaces, such as in electroplating. By controlling the composition of the solution and the potential difference applied between the electrodes, specific metals can be deposited onto a desired surface.
learn more about solution
https://brainly.com/question/11632115
#SPJ11
Give the number of protons and the number of neutrons in the nucleus of the following isotopes: a) Carbon-14 b) Cobalt-60 c) Gold-197 d) Uranium-235
Answers
Explanation:
We are given different isotopes and we have to identify the number of protons and neutrons that are in the nuclueus of each atom.
a) Carbon-14:
By definition two isotopes are atoms that have the same atomic number but different mass number. The atomic number of an atom is equal to the number of protons of that atom, and the mass number is equal to the number of protons plus the number of neutrons.
atomic number = n° of protons
mass number = n° of protons + n° of neutrons
n° of protons = atomic number
n° of neutrons = mass number - n° of protons
n° of neutrons = mass number - atomic number
If two isotopes have the same atomic number but different mass number we can say that two isotopes have the same number of protons but different number of neutrons.
In we pay attention to carbon-14 we can look for its atomic number in the period table: 6. And its mass number is the one that we are given after the name of the element: 14.
n° of protons = atomic number = 6
n° of protons = 6
n° of neutrons = mass number - atomic number = 14 - 6
n° of neutrons = 8
b) Cobalt-60:
atomic number = 27 (from the periodic table)
mass number = 60
n° of protons = atomic number = 27
n° of protons = 27
n° of neutrons = mass number - atomic number = 60 - 27
n° of neutrons = 33
c) Gold-197:
atomic number = 79 (from the periodic table)
mass number = 197
n° of protons = atomic number = 79
n° of protons = 79
n° of neutrons = mass number - atomic number = 197 - 79
n° of neutrons = 118
d) Uranium-235:
atomic number = 92 (from the periodic table)
mass number = 235
n° of protons = atomic number = 92
n° of protons = 92
n° of neutrons = mass number - atomic number = 235 - 92
n° of neutrons = 143
Answer:
a) Carbon-14: n° of protons = 6 n° of neutrons = 8
b) Cobalt-60: n° of protons = 27 n° of neutrons = 33
c) Gold-197: n° of protons = 79 n° of neutrons = 118
d) Uranium-235: n° of protons = 92 n° of neutrons = 143
Which of these molecular geometries forms a molecule with a bent shape?
A. linear geometry with two lone pairs of electrons
B. tetrahedral geometry with one lone pair of electrons
C. trigonal planar geometry with one lone pair of electrons
D. trigonal planar geometry with two lone pairs of electrons
Answers
The molecular geometries forms a molecule with a bent shape is linear geometry with two lone pairs of electrons.
Molecular geometries often deals with the number of atoms and the number of lone pair electrons.The main geometries without lone pair electrons are: linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral
bent electron geometry?
The bent geometry are the final 4 electron group geometry. This takes place when there are 2 bonds and 2 lone pairs.
Linear is known to be equal to the line of atoms with a 180° angle. it's 2 or 3 atoms total.
Bent is often equal to Linear but bent as a result of the Lone Pairs that it contains,
The more the Lone Pairs, the greater the bent and the smaller the degree. It is therefore 3 atoms total.
Learn more from
https://brainly.com/question/16049749
name the three types of rain fall
Answers
Explanation:
Convectional rainfall.
Orographic or relief rainfall.
Cyclonic or frontal rainfall
Answer:
Hey mate....
Explanation:
This is ur answer....
The 3 types of rainfall are Convectional, Orographic and Cyclonic.....it is same as Relief Rainfall, Convectional Rainfall and Frontal Rainfall.Hope it helps you!
Mark me as the brainliest....
FOLLOW ME! :)
1. lead has an average atomic mass of 207.19 amu. the three major isotopes of lead are pb-206 (205.98 amu); pb-207 (206.98 amu); and pb-208 (207.98 amu). if the isotopes of pb-207 and pb-208 are present in equal amounts, calculate the percent abundance of pb-206, pb-207, pb-208.
Answers
Percent abundance of the three major isotopes of lead are Pb-206: 19.34%, Pb-207: 40.33% ,Pb-208: 40.33%
What is an isotope ?Isotopes are atoms that have the same number of protons but differing numbers of neutrons. They have nearly identical chemical properties but differ in mass and thus in physical qualities.If w(Pb-207) = w(Pb-208) = x (equal amounts),
then:
w(Pb-206) = 1 - w(Pb-207) - w(Pb-208) = 1 - x - x = 1-2x.
i.e.
[w(Pb-206) × Isotopic mass of Pb-206] + [w(Pb-207) × Isotopic mass of Pb-207] + [w(Pb-208) × Isotopic mass of Pb-208] = Average atomic mass of Pb
(1-2x)(205.98) + (x)(206.98) + (x)(207.98) = 207.19
205.98 - 411.96x + 206.98x + 207.98x = 207.19
3x = 1.21
x = 0.4033
w(Pb-207) = w(Pb-208) = x = 0.4033 or 40.33%
w(Pb-206) = 1-2x = 1 - 2×0.4033 = 0.1934 or 19.34%
Pb-206: 19.34%
Pb-207: 40.33%
Pb-208: 40.33%
To learn more about isotope refer,
https://brainly.com/question/14220416
#SPJ4
ASAP!!MARK BRAINLIEST! Mia wants to get her drinking water from an artesian well on her property. To make sure
that this water is drinkable, she must have a laboratory analyze it. Canadian standards state that the maximum permitted amount of boron in drinking water is 5 ppm. A laboratory analyzed a sample of 250 mL of water from Mia's well. This sample
contained 0.1 mg of boron.
Can Mia drink the water from her well?
O Yes
O No
Answers
Answer: yes
Explanation:
Answer:
yes
Explanation:
due to the properties of baron she should be fine
WILL MARK AS BRAINLIEST IF CORRECT

Answers
Answer:
I don't know what is the actual answer
14. All living things react to sound, touch etc. Whatever causes a living thing to react is called a stimulus. The reaction is called a response. Which of these is an example of a STIMULUS- RESPONSE?
Answers
in the thermodynamics experiment, why was it necessary to filter the hot calcium hydroxide solution?
Answers
In a thermodynamics experiment, filtering the hot calcium hydroxide solution is necessary to removes any impurities or particulates that may be present in the solution that can affect the outcome of the experiment by introducing errors in the results.
In addition to this, filtering the hot calcium hydroxide solution also helps to ensure that the concentration of the solution remains consistent throughout the experiment. This is important because the concentration of the solution affects the thermodynamic properties of the solution, such as the temperature at which the reaction occurs. Without filtering, the concentration would vary depending on the amount of impurities present, and this could lead to inaccurate results.
Finally, filtering the hot calcium hydroxide solution also helps to prevent clogs or blockages in the equipment used for the experiment. If impurities are able to pass through the equipment, it could cause a disruption in the flow of the solution, which could affect the accuracy of the experiment.
Learn more about thermodynamics at :https://brainly.com/question/1604031
#SPJ4
most of the elements on the periodic table are produced by the expanding shockwave from a type ii supernova. this process is known as group of answer choices nuclear fusion nuclear fusion stellar nucleosynthesis exploding (supernova) nucleosynthesis
Answers
Most of the elements on the periodic table are produced by the expanding shockwave from a type ii supernova. this process is known as nucleosynthesis.
In the centres of most stars, lighter elements like hydrogen and helium are fused to form the most prevalent elements, such as carbon and nitrogen. However, only large stars that die in supernova explosions may produce the strongest elements, including iron.
Large nuclear reactors make up stars. Massive atomic collisions that tear apart atoms in the centre of stars change their atomic structure and unleash a great amount of energy. The result is hot and brilliant stars. Stars are powered by nuclear fusion, an atomic reaction. Because they are so strong, supernovae produce brand-new atomic nuclei. When a big star collapses, a shockwave is created that may trigger fusion reactions in the star's outer shell. Nucleosynthesis, a process that results from these fusion processes, produces fresh atomic nuclei.
To know more about nucleosynthesis here
https://brainly.com/question/28517064
#SPJ4
Which of the following substances will increase the molar solubility of nickel(II) phosphate in a saturated solution?
a. (NH4)3PO4
b. NH4Cl
c. Na3PO4
d. KOH
e. HNO3
Answers
The substance that will increase the molar solubility of nickel(II) phosphate in a saturated solution is c. Na3PO4 (sodium phosphate).
To determine the substance that will increase the molar solubility of nickel(II) phosphate, we need to consider the common ion effect and the solubility product principle.
Nickel(II) phosphate has a chemical formula of Ni3(PO4)2, and its solubility can be affected by the presence of other ions in solution. In this case, we are looking for a substance that will introduce an ion that can form a more soluble compound with one of the ions in nickel(II) phosphate.
Among the given substances, Na3PO4 is the only one that introduces an ion (PO4^3-) that can form a more soluble compound with one of the ions in nickel(II) phosphate. When sodium phosphate is added to a saturated solution of nickel(II) phosphate, the PO4^3- ions will combine with the Ni^2+ ions to form the more soluble compound sodium nickel phosphate (Na2Ni(PO4)2).
The other substances (a. (NH4)3PO4, b. NH4Cl, d. KOH, and e. HNO3) do not introduce ions that can form more soluble compounds with the ions in nickel(II) phosphate. Therefore, they will not increase the molar solubility of nickel(II) phosphate.
Among the given substances, sodium phosphate (Na3PO4) is the only one that will increase the molar solubility of nickel(II) phosphate in a saturated solution.
Sodium phosphate introduces PO4^3- ions, which can combine with the Ni^2+ ions to form a more soluble compound. The other substances do not have ions that can form more soluble compounds with nickel(II) phosphate.
Learn more about molar solubility here https://brainly.com/question/31488311
#SPJ11
16 - A sample of hydrogen gas collected by displacement of water occupied 30.0 mL at 24 °C on a day when the barometric pressure was 736 mmHg. What volume would the hydrogen occupy if it were dry and at STP? (The vapor pressure of water at 24.0 °C is 22.4 mmHg.) a) 21.65 mL b) 0 27.6 mL c) 36.84 mL d) 25.83 mL e) 32.4 mL
Answers
The volume of hydrogen gas, if it were dry and at STP, would be approximately 19030 mL. None of the provided answer options match this value, so none of them are correct.
To determine the volume of hydrogen gas if it were dry and at STP (standard temperature and pressure), we need to apply the ideal gas law. The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure of the gas
V = volume of the gas
n = number of moles of the gas
R = ideal gas constant (0.0821 L·atm/mol·K)
T = temperature of the gas in Kelvin
First, let's convert the given temperature of 24 °C to Kelvin:
T = 24 °C + 273.15 = 297.15 K
Next, we need to determine the number of moles of hydrogen gas. To do this, we use the concept of partial pressure.
The partial pressure of hydrogen gas (PH2) can be calculated by subtracting the vapor pressure of water (Pwater) from the total pressure (Ptotal):
PH2 = Ptotal - Pwater
Ptotal = 736 mmHg
Pwater = 22.4 mmHg (vapor pressure of water at 24.0 °C)
PH2 = 736 mmHg - 22.4 mmHg = 713.6 mmHg
Now we can rearrange the ideal gas law equation to solve for the volume (V):
V = (nRT) / P
To find the number of moles (n), we can use the ideal gas law equation with the given conditions:
n = (PV) / (RT)
Substituting the values:
n = (713.6 mmHg * 30.0 mL) / (0.0821 L·atm/mol·K * 297.15 K)
Now we can calculate the number of moles (n).
n = (713.6 mmHg * 30.0 mL) / (0.0821 L·atm/mol·K * 297.15 K)
≈ 0.8418 mol
Now, we can substitute the values of n, R, and T into the equation V = (nRT) / P to calculate the volume (V) at STP:
V = (0.8418 mol * 0.0821 L·atm/mol·K * 273.15 K) / (1 atm)
≈ 19.03 L
Finally, we need to convert the volume from liters to milliliters:
V = 19.03 L * 1000 mL/L
≈ 19030 mL
Learn more about vapor at: brainly.com/question/32499566
#SPJ11
You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100M benzoic acid (pK
a
=4.20) and 0.240M sodium benzoatc. How many milliliters of each solution should be mixed to prepare this buffer? benzoic acid:
Previous question
Answers
To prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.The ratio of benzoic acid to sodium benzoate in the buffer solution using the Henderson-Hasselbalch equation.
To prepare a pH 4.00 buffer solution using benzoic acid and sodium benzoate, we need to calculate the appropriate volumes of the 0.100 M benzoic acid and 0.240 M sodium benzoate solutions.
First, we need to determine the ratio of benzoic acid to sodium benzoate in the buffer solution. The Henderson-Hasselbalch equation can help us with this calculation:
pH = pKa + log([A-]/[HA])
Given that the pH is 4.00 and pKa is 4.20, we can rearrange the equation:
log([A-]/[HA]) = pH - pKa
log([A-]/[HA]) = 4.00 - 4.20
log([A-]/[HA]) = -0.20
Next, we take the antilog of -0.20 to find the ratio of [A-] to [HA]:
[A-]/[HA] = antilog(-0.20)
[A-]/[HA] = 0.63
The ratio of [A-] to [HA] is 0.63.
Now, let's calculate the volumes of each solution needed. Let's assume x represents the volume (in mL) of the 0.100 M benzoic acid solution and y represents the volume (in mL) of the 0.240 M sodium benzoate solution.
Since the total volume is 100.0 mL, we have the equation: x + y = 100
Considering the ratio of [A-] to [HA] as 0.63, we can write the equation: y/x = 0.63
Solving these two equations simultaneously will give us the volumes of each solution:
x + y = 100
y/x = 0.63
By substituting y = 0.63x from the second equation into the first equation, we get:
x + 0.63x = 100
1.63x = 100
x = 61.35 mL (rounded to two decimal places)
Substituting this value back into the equation x + y = 100, we find:
61.35 + y = 100
y = 38.65 mL (rounded to two decimal places)
Therefore, to prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.
To know more about the Henderson-Hasselbalch equation, click here, https://brainly.com/question/31732200
#SPJ11
How is it possible for water to exist as a solid, liquid, and gas all in the same place?
Answers
Answer:
Explanation: Take, for example, an ice cube in an open or closed container, that is in the process of melting at room temperature. There is solid water, liquid water, and water vapour (gas) in the air.
a sample of seawater from a tidal estuary was found to contain a concentration of 631 mg of chloride ions per kg of seawater. if the density of the sample was 1.035 g/mL what is the molarity of the chloride ion
Answers
The molarity of the chloride ion in the seawater sample is 19.6 M.
Concentration of chloride ions = 631 mg/kg of seawater
Density of sample = 1.035 g/mL
We need to calculate the molarity of the chloride ion. The formula to calculate molarity is:
Molarity = moles of solute / volume of solution in liters
To calculate the moles of chloride ions in the sample, we first need to convert the concentration from mg/kg to g/L.
Concentration in g/L = (631 mg/kg * 1 kg / 1000 g) = 0.631 g/L
Now we need to find the volume of the solution in liters.
We know that the density of the sample is 1.035 g/mL. So the mass of 1 L of sample will be:
mass = density * volume = 1.035 g/mL * 1000 mL = 1035 g
We can now convert the mass of the sample to volume using the density:
volume = mass / density = 1035 g / 1.035 g/mL = 999.03 mL = 0.99903 L
Now we can calculate the moles of chloride ions:
moles = concentration * volume = 0.631 g/L * 0.99903 L = 0.6301 mol
Finally, we can calculate the molarity of chloride ions:
Molarity = moles / volume = 0.6301 mol / 0.032 L = 19.6 M
Therefore, the molarity of the chloride ion in the seawater sample is 19.6 M.
Learn more about molarity here:
https://brainly.com/question/31545539
#SPJ11
how many grams of oxygen are produced when 6.06 g of potassium chlorate decompose completely
Answers
Answer:
2.38 g of oxygen (O2).
Explanation:
What is given?
Mass of potassium chlorate (KClO3) = 6.06 g.
Molar mass of KClO3 = 122.4 g/mol.
Molar mass of oxygen (O2) = 32 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation. Remember that the decomposition of a compound produces two or more products:
\(2KClO_3\rightarrow2KCl+3O_2.\)Now, let's convert 6.06 g of KClO3 to moles using its molar mass:
\(6.06\text{ g KClO}_3\cdot\frac{1\text{ mol KClO}_3}{122.4\text{ g KClO}_3}=0.0495\text{ moles KClO}_3.\)You can see in the chemical equation that 2 moles of KClO3 produce 3 moles of O2. By doing a rule of three with this data, we obtain that:
\(0.0495\text{ moles KClO}_3\cdot\frac{3\text{ moles O}_2}{2\text{ moles KClO}_3}=0.0743\text{ moles O}_2.\)The final step is to convert from 0.0743 moles of O2 to grams using its molar mass, like this:
\(0.0743\text{ moles O}_2\cdot\frac{32\text{ g O}_2}{1\text{ }mol\text{ }O_2}=2.38\text{ g O}_2.\)The answer is that we will produce 2.38 g of oxygen (O2) from the decomposition of 6.06 g of potassium chlorate (KClO3).
Using the phase diagram for H2O what phase is water in at 1 atm pressure and -5C

Answers
The phase diagram of water depicts the behavior of water with respect to temperature and pressure, showing the physical states of water: solid, liquid, and gas, at different points on the diagram. It is also known as the pressure-temperature phase diagram
Water’s phase diagram has three phases, ice (solid), water (liquid), and steam (gas), which exist in equilibrium at the normal atmospheric pressure of one atmosphere (1 atm).At 1 atm pressure and -5°C, water is in a solid state, which is ice. The horizontal line on the diagram at 1 atm represents the normal atmospheric pressure on earth, while the vertical line at -5°C depicts the temperature point where the phase transition between water and ice occurs. The intersection of the horizontal and vertical lines indicates the phase of water at that specific temperature and pressure. When water is heated at 1 atm, its temperature increases until it reaches 100°C, where it boils and turns into steam (gas). Similarly, when water is cooled, its temperature decreases until it reaches 0°C, where it freezes and becomes ice (solid).When water is at 1 atm and at a temperature between 0°C and 100°C, it exists in a liquid state. If the temperature and pressure change, the physical state of water changes as well. Hence, the phase diagram of water helps us understand the behavior of water at different temperatures and pressures.
For such more question on temperature
https://brainly.com/question/27944554
#SPJ8
Which of the following chemical equations is connected to the definitions of:
Part A
the first ionization energy of nitrogen
A. N+(g)→N2+(g)+e−
B.N(g)+e−→N−(g)
C. N(g)→N2+(g)+2e−
D. N(g)→N+(g)+e−
E. N(g)+2e−→N2−(g)
Answers
The amount of energy needed to remove an electron from a neutral gaseous atom is known as ionization energy. The gaseous atom gains positive charge and loses an electron when we apply this energy.
The correct answer is N(g)→N+(g)+e−.
What is Ionization energy?
Ionization energy can be defined simply as a measurement of how difficult it is to remove an electron from an atom or ion or of an atom's or ion's propensity to give up an electron. Usually, when a chemical species is in its ground state, an electron is lost.
As an alternative, we might say that ionization or ionisation energy is a measurement of the intensity of the attractive forces that hold an electron in a specific location.
To learn more about Ionization energy visit;
https://brainly.com/question/27356170
#SPJ4
Kinetic Molecular Theory and the Ideal Gas LawsMPREHENSION
Professor Dave Explains
(press pause for more time)
A 5.15L balloon has a pressure of 1.35 atm. If compressed to 3.43L,
what will be the resulting pressure?
C
0.785 moles of N₂ fill a balloon at 1.5 atm and 301 K.
What is the volume of the balloon?
Please explain how you got the answer. I don’t really understand ideal gas and it’s laws. Please Help!!!
Thank you so much
Answers
The pressure of A 5.15L balloon has a pressure of 1.35 atm. If compressed to 3.43L is 2.03 atm and volume of 0.785 moles of N₂ fill a balloon at 1.5 atm and 301 K is 19.4 L.
How to calculate pressure?According to the equation of ideal gas
\(P_{1} V_{1} =P_{2} V_{2}\)
Substituting the value in the above equation,
\(1.35*5.15=P_{2} *3.43\)
\(P_{2} =\frac{1.35*5.15}{3.43}\)
\(P_{2}\)= 2.03 atm
How to calculate volume?According to ideal gas equation
PV=nRT
Substituting value in above equation
\(V= \frac{nRT}{P}\)
\(V= \frac{0.785*0.0821*301}{1.5}\)
V= 19.4 L
The equation of state of an ideal gas, often known as the general gas equation, is the ideal gas law. Even though it has a number of restrictions, it is a good approximation of the behavior of various gases under a variety of situations.
For more information on ideal gas equation kindly visit to
https://brainly.com/question/4147359
#SPJ1
Answer question number 3

Answers
Answer:
A
Explanation:
A mixture is formed when two or more substances are physically mixed together. A compound is formed when two or more substances are chemically combined through a chemical reaction.
What mass of aluminum can be plated onto an object in 750 minutes at 5. 57 a of current?
Answers
To determine the mass of aluminum that can be plated onto an object in 750 minutes at a current of 5.57 A, we need to use the formula for electroplating.
The formula is: Mass of metal plated = (Current × Time × Atomic mass of metal) / (Faraday's constant × Valency of metal)
In this case, we are plating aluminum onto the object. The atomic mass of aluminum is 26.98 g/mol, the valency is 3, and the Faraday's constant is 96,485 C/mol.
Plugging these values into the formula, we get:
Mass of aluminum plated = (5.57 A × 750 minutes × 26.98 g/mol) / (96,485 C/mol × 3)
Calculating this equation will give us the mass of aluminum plated onto the object.
However, it's important to note that we can't calculate this accurately without knowing the surface area of the object to be plated and the efficiency of the electroplating process. The formula assumes 100% efficiency, which is not always the case in real-world situations.
To know more about mass of aluminum visit:
brainly.com/question/13908418
#SPJ11