determine the freezing point of a solution which contains 0.22 mol of fructose in 145 g of water. (for water, kf = 1.86°c/m)
Answers
The freezing point of the solution is -2.83°C.
To determine the freezing point of the solution containing 0.22 mol of fructose in 145 g of water, you can use the formula:
ΔTf = Kf × molality
where ΔTf is the change in freezing point, Kf is the freezing point depression constant for water (1.86°C/m), and molality is the concentration of the solution in mol/kg.
First, calculate the molality (mol solute/kg solvent):
Molality = moles of solute / mass of solvent (in kg)
The mass of water in kg is 145 g / 1000 = 0.145 kg
Moles of fructose = 0.22 mol
Putting, the values in the equation;
Molality = (0.22 mol) / (145 g * 1 kg/1000 g)
= 0.22 mol / 0.145 kg
= 1.52 mol/kg
Next, use the formula with the given Kf for water (1.86°C/m):
ΔTf = (1.86°C/m) * (1.52 mol/kg)
= 2.83°C
Therefore, the freezing point of the solution is lowered by 2.83°C. To find the actual freezing point, we need to subtract this value from the freezing point of pure water (0°C):
Freezing point of the solution = 0°C - 2.83°C
= -2.83°C
To know more about the molal freezing point constant, click below.
https://brainly.com/question/12785573
#SPJ11
Related Questions
an object emits a range of electromagnetic energy wave lengths because
Answers
An object emits a range of electromagnetic energy wavelengths because different energy levels of its constituent particles undergo transitions and emit photons with varying energies.
The emission of a range of electromagnetic energy wavelengths by an object can be explained by the behavior of its constituent particles, such as atoms or molecules. These particles possess different energy levels, and when they undergo transitions between these energy levels, they emit photons of corresponding energies.
In an atom, for example, electrons occupy specific energy levels or orbitals. When an electron transitions from a higher energy level to a lower energy level, it releases energy in the form of a photon. The energy of the emitted photon corresponds to the energy difference between the initial and final energy levels of the electron. Since atoms have multiple energy levels, electrons can transition between various combinations of energy levels, resulting in the emission of photons with different wavelengths.
Similarly, molecules can exhibit vibrational, rotational, and electronic energy levels. Transitions between these levels can lead to the emission of photons across a range of wavelengths..
learn more about electromagnetic energy here:
https://brainly.com/question/14824974
#SPJ4
the complete question is:
Why does an object emit a range of electromagnetic energy wavelengths?
what is the advantage of rusting
Answers
Answer:
Provide more strength
Explanation:
Rust is metal that has been oxidised . Oxides are usually more fragile and porous than their crystals metal equivalents. Some oxides, such as Aluminum oxide, are useful because they have a thin, strong shell that protects the metal from further corrosion.
Atoms are the building blocks for

Answers
Answer:all matter.
Explanation:
-Love Ricardo H. Sparks
Answer:
Atoms are the building blocks of life (everything basically)
Explanation:
The temperature of a 0.65L sample of carbon dioxide gas is 580K. If the pressure remains constant, what is the new volume of the gas if the temperature increases to 1300K?
Answers
Which set of elements contains a metalloid?
Oa. Ba, Ag, Sn, Xe
O b. Fr, F, O, Rn
O c. Li, Mg, Ca, Kr
O d. K, Mn, As, Ar
Answers
Answer:
D.) K, Mn, As, Ar
Explanation:
The metalloids are located in the p-block on the periodic table and have a ladder-like arrangement.
The most commonly recognized metalloids are:
boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te)
In another experiment the student turreted 50.0
Answers
Answer:
is this a full question ????
Explanation:
how many total elements are in marble?
Answers
Answer:There are total 118 elements in world as about marbale
Explanation:

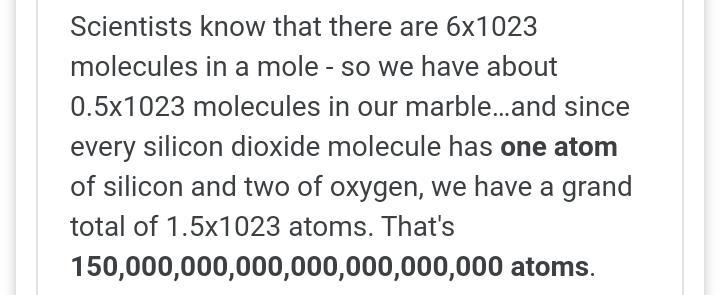
2. convert 4.22 cL to mL
Answers
Answer:
1 cl =10 ml
so
4.22cL= 4.22×10= 42.2 mL
A 2.5l container has a gas of 4.6atm. If the volume is decreased to 1.6l. What will be the new pressure inside the container
Answers
Answer:
Just add and multiply after divding and lol
Explanation: add then add
Suppose that 3.33 g of acetone at 25.0 °C condenses on the surface of a 44.0-g block of aluminum that is initially at 25 °C. If the heat released during condensation goes only toward heating the metal, what is the final temperature (in °C) of the metal block?
Answers
Answer:
68.6 °C
Explanation:
From conservation of energy, the heat lost by acetone, Q = heat gained by aluminum, Q'
Q = Q'
Q = mL where Q = latent heat of vaporization of acetone, m = mass of acetone = 3.33 g and L = specific latent heat of vaporization of acetone = 518 J/g
Q' = m'c(θ₂ - θ₁) where m' = mass of aluminum = 44.0 g, c = specific heat capacity of aluminum = 0.9 J/g°C, θ₁ = initial temperature of aluminum = 25°C and θ₂ = final temperature of aluminum = unknown
So, mL = m'c(θ₂ - θ₁)
θ₂ - θ₁ = mL/m'c
θ₂ = mL/m'c + θ₁
substituting the values of the variables into the equation, we have
θ₂ = 3.33 g × 518 J/g/(44.0 g × 0.9 J/g°C) + 25 °C
θ₂ = 1724.94 J/(39.6 J/°C) + 25 °C
θ₂ = 43.56 °C + 25 °C
θ₂ = 68.56 °C
θ₂ ≅ 68.6 °C
So, the final temperature (in °C) of the metal block is 68.6 °C.
The final temperature of the metal block is 74.97°C
What is the specific heat?The specific heat of a substance is the required quantity of heat needed to raise the temperature of 1 gram of the substance by 1° C.
From the parameters given:
The mass of acetone = 3.33 gThe number of moles of acetone is:
\(\mathbf{= 3.33 g \times \dfrac{mol}{58.08 \ mol}}\)
= 0.0573 mol
At standard conditions, the heat of vaporization of acetone is:
\(\mathbf{\Delta H = 32.0 \ kJ/mol \times 0.0578 \ mol } \\ \\ \mathbf{\Delta H = 1.8496 \ kJ } \\ \\ \mathbf{ \Delta H = 1.85 \times 10^3 \ J}\)
Given that:
The mass of the metal (m) = 44.0 gThe initial temperature \(\mathbf{T_1}\) = 25° CThe final temperature \(\mathbf{T_2 = ???}\)The specific heat of the aluminum is = 0.903 J/g° C
The heat energy can be computed as:
q = msΔT
q = 41 g × 0.903 J/g° C × (x - 25°C)
Using the calorimetry principle, heat energy lost by metal = heat energy gained by acetone.
i.e.
\(\mathbf{q_{(acetone)} gain = q_{(metal)} lost }\)
\(\mathbf{-1.85 \times 10^3 \ J = - 41 g \times 0.903 \ J/g^0 C \times ( x - 25^0 c) }\)
\(\mathbf{1.85 \times 10^3 \ J = 41 g \times 0.903 \ J/g^0 C \times ( x - 25^0 c) }\)
\(\mathbf{(x - 25 ^0 C) = \dfrac{1.85 \times 10^3 \ J }{ 41 g \times 0.903 \ J/g^0 C}}\)
\(\mathbf{(x - 25 ^0 C) = 49.97^0 C}}\)
\(\mathbf{x = 49.97^0 C+25 ^0 C}}\)
x = 74.97 °C
Learn more about specific heat here:
https://brainly.com/question/1430612
8. Numerical problems a. If the relative density of gold metal is 19, find its density in SI unit. Given, density of water at 4°C is 1000 kg/m³.
Answers
The density of gold in SI units is 19,000 kg/m³. Gold's relative density of 19 means that it is 19 times denser than water at 4°C, which has a density of 1000 kg/m³. Therefore, by multiplying the relative density of gold by the density of water, we can find the density of gold in SI units. In this case, 19 multiplied by 1000 kg/m³ equals 19,000 kg/m³, which is the density of gold in SI units.
In the context of this problem, the relative density of a substance is defined as the ratio of its density to the density of a reference substance, in this case, water at 4°C. By multiplying the relative density by the density of the reference substance, we can calculate the density of the substance in SI units.
In this example, the relative density of gold is 19, indicating that gold is 19 times denser than water at 4°C. Multiplying the relative density by the density of water (1000 kg/m³) yields the density of gold as 19,000 kg/m³ in SI units.
To summarize, the density of gold in SI units is 19,000 kg/m³. The relative density of gold is 19, indicating that it is 19 times denser than water at 4°C, which has a density of 1000 kg/m³.
for such more questions on density
https://brainly.com/question/26364788
#SPJ8
What are two qualities of nonmetals? Describe each
Answers
Answer:
In the elemental form, non-metals can be gas, liquid or solid. They aren't shiny (lustrous) and they don't conduct heat or electricity well. Usually their melting points are lower than for metals, although there are exceptions. The solids usually break easily, and can't bend like metals
Explanation:
All plants and animals use the process of choose. To obtain energy from food. This process begins in the choose. , where glucose is broken down into smaller molecules to produce energy. The second part of the process takes place in the choose. . Here the molecules are broken down further and produce choose. As a waste product. In addition, the second part of the process requires choose. In order to function.
Answers
Oxygen can be present or absent during cellular respiration. Because the cell appears to breathe in molecular oxygen and exhale carbon dioxide, the process is known as cellular respiration.
What are molecules called?Any atoms joined by chemical bonds are referred to be molecules in general. A molecule is made up of any two atoms together. A molecule consisting of atoms from various elements is referred to as a compound. Although all molecules are compounds, not all compounds are molecules.
What three molecules are necessary for all life?Nucleic acids, lipids, proteins, and carbohydrates make up the four basic building blocks of life. Each and every one of the 4 categories is essential for every living thing on Earth. A cell or organism could not survive without any one of these four molecules.
To know more about Molecules visit:
https://brainly.com/question/19556990
#SPJ4
Calculate the pH of each of the following solutions at 25°C. a. 0.140 M HONH2 (Kb = 1.1 x 10-8) pH = b. 0.140 M HONH3 C1 pH = c. pure H2O pH = d. a mixture containing 0.140 M HONH, and 0.140 M HONH3CI pH =
Answers
The pH of each of the following solutions at 25°C will be :
a. 0.140 M HONH2 (Kb = 1.1 x 10-8), pH =10.98
b. 0.140 M HONH3 C1, pH = 1
c. pure H2O ,pH = 7
d. a mixture containing 0.140 M HONH, and 0.140 M HONH3CI, pH = 5.05
Define pH
The pH scale determines how acidic or basic water is. The range is 0 to 14, with 7 representing neutrality. Acidity is indicated by pH values below 7, whereas baseness is shown by pH values above 7. In reality, pH is a measurement of the proportion of free hydrogen and hydroxyl ions in water.
a. 0.140 M HONH2 (Kb = 1.1 x 10-8)
Kb = [OH-][HONH2]/[NH2OH]
1.1 x 10^-8 = [OH-][0.100]/[NH2OH]
[NH2OH] = [HONH2] = 0.100 M
1.1 x 10^-8 = [OH-]^2/0.100
[OH-] = sqrt(1.1 x 10^-9) = 1.05 x 10^-5 M
pH = 14 - pOH = 14 + log[OH-] = 14 + log(1.05 x 10^-5) ≈ 10.98
b. 0.140 M HONH3 C1
HONH3Cl → H+ + ONH3Cl-
[H+] = 0.100 M
pH = -log[H+] = -log(0.100) = 1.00
c. Pure water has a neutral pH of 7.00.
d. a mixture containing 0.140 M HONH, and 0.140 M HONH3CI
pH = pKa + log([A-]/[HA])
The pKa of HONH3+ is 14 - pKb = 14 - 8.95 = 5.05
[A-]/[HA] = [HONH2]/[HONH3+] = 0.100/0.100 = 1
pH = 5.05 + log(1) = 5.05
To learn more about pH :
https://brainly.com/question/172153
#SPJ4
QUIZ 4: GOLDEN YEARS TO IONIZATION The elements with the highest ionization energy and thus the most unreactive are:
Answers
The elements with the highest ionization energy are the noble gases, which are located in the upper right corner of the periodic table. These elements include helium, neon, argon, krypton, xenon, and radon. These elements have a full valence electron shell, meaning that they have the maximum number of electrons possible in their outermost energy level. This makes them very stable and resistant to chemical reactions, as they do not have any electrons that can be easily removed or added.
As a result, they have very high ionization energy, meaning that a large amount of energy is required to remove an electron and form an ion. Due to these properties, these elements are not readily reactive with other elements and tend to exist as diatomic molecules or as individual atoms. These elements are used in a variety of industrial, medical, and everyday applications such as in lighting, refrigeration, and as inert gases in medical equipment and in the manufacturing of semiconductors.
Learn more about Ionisation Energy here: https://brainly.com/question/27356170
#SPJ4
PLEASE URGENT HELP!! 30 POINTS!!!!!!

Answers
Answer:
CH³–C=CH——CH³–CH=CH²
Explanation:
THIS IS PROPYNE TO PROPENE
1. why did you perform atomic emission analysis on the sample that contained both khp and kcl?
Answers
Atomic emission analysis was performed on the sample containing both KHP (potassium hydrogen phthalate) and KCl (potassium chloride) to determine the concentrations of the individual components in the sample.
Atomic emission refers to the process where atoms in a sample are excited by an external energy source, such as heat or electricity. When the excited atoms return to their ground state, they emit light with specific wavelengths characteristic of the elements present in the sample. By analyzing the emitted light's wavelength and intensity, we can identify and quantify the elements in the sample. In the case of KHP and KCl, atomic emission analysis was used to determine the concentrations of potassium (K), as well as any other elements that might be present. This information is essential in various applications, such as quality control, environmental monitoring, and chemical analysis. By obtaining accurate concentration data, you can ensure the sample's proper composition and make informed decisions regarding its use and potential impact on the environment or other processes.
To know more about Atomic emission
https://brainly.com/question/29255944
#SPJ11
A 0.520 g sample of an unknown nonelectrolyte compound is dissolved in 4.12 g of lauric acid (Kf = 3.90 °C/m). The freezing point depression is determine to be 4.20 °C. What is the molar mass of the compound?
Answers
The molar mass of the unknown nonelectrolyte compound is
Using the formula;
∆T = K m i
Where;
K = freezing point depression constant
m = molality of the solution
i = Van't Hoft factor
Note that i = 1 since the compound is a nonelectrolyte.
To find molality;
Number of moles = 0.520 g/Molar mass
Let the molar mass of the unknown compound be MM
Number of moles = 0.520 g/MM
Number of kilograms of solvent = 4.12 g/1000 = 0.00412 Kg
Molality = 0.520 g/MM * 1/0.00412 Kg
Freezing point depression is 4.20 °C
To find the molar mass of the compound;
4.20 °C = 3.90 °C/m * 0.520 g/MM * 1/0.00412 Kg
4.20 = 492.23/MM
MM = 492.23/4.20
MM = 117.19 g/mol
Learn more; https://brainly.com/question/14762341
While molecules such as glucose move freely in the blood, triglycerides are carried in the blood by very low density lipoproteins (VLDLs) in the form of chylomicrons. A chylomicron resembles a droplet, with phospholipid heads and VLDLs facing water, the solvent of blood, while triglycerides are found within. The outer surface resembles that of a regular phospholipid bilayer. If the contents of a chylomicron are all lipids
A. then they should be soluble in blood plasma.
B. an inner layer is needed because phospholipids always form bilayers,
C. there is no need for an inner phospholipid layer in a chylomicron
Answers
Answer:
B. an inner layer is needed because phospholipids always form bilayers.
Explanation:
Lipids are organic compounds which are not soluble in water but they are soluble in organic compounds. They are formed in body by combination of various molecules. The inner layer of phospholipids always form bilayers.
Chlorine gas reacts with aqueous sodium bromide to produce aqueous sodium chloride and bromine gas. What is the balanced net ionic equation for this reaction?
Cl2(g) + 2 Br1-(aq) → 2 Cl1-(aq) + Br2(g)
Cl2(g) + 2 Na1+(aq) + 2 Br1-(aq) → 2 Na1+(aq) + 2 Cl1-(aq) + Br2(g)
2 Cl1-(g) + 2 Na1+(aq) + 2 Br1-(aq) → 2 Na1+(aq) + 2 Cl1-(aq) + 2 Br1-(g)
Cl2(g) + 2 NaBr(aq) → 2 NaCl(aq) + Br2(g)

Answers
Answer:
Explanation:
Whole formula equation:
Cl₂(g) + 2NaBr(aq) → 2NaCl(aq) + Br₂(g)
We can expand this to get the full ionic equation:
Cl₂(g) + 2Na⁺(aq) + 2Br⁻(aq) → 2Na⁺(aq) + 2Cl⁻(aq) + Br₂(g)
Cancelling out spectator ions (ions that don't change) on both sides:
Cl₂(g) + 2Na⁺(aq) + 2Br⁻(aq) → 2Na⁺(aq) + 2Cl⁻(aq) + Br₂(g)
And our final result, is the net ionic equation:
Cl₂(g) + 2Br⁻(aq) → 2Cl⁻(aq) + Br₂(g)
What u learned about the states of matter?
Answers
Answer:t
Explanation:
In everyday life, four states of matter are visible: solid, liquid, gas, and plasma.
Of course, matter also has other states, such as Bose-Einstein density and Fermion density, but these states are only observed under certain conditions.
Use the solubility curve to match each scenario with its correct saturation level. All scenarios are in 100g of water.

Answers
The curve represents saturation. Below the curve, the water is unsaturated. Above the curve, water is supersaturated. This means that more solute is present than the water can contain.
The line of the solubility curve indicates that the solution is saturated. A saturated solution is defined as a solution in which 100 g of solute is dissolved in 100 g of water. Simulations below this line indicate unsaturated solutions.
The difference between unsaturated and saturated solutes can be determined by adding very small amounts of solute to the solution. In unsaturated solutes, solutes will dissolve, and solutes in saturated solutes will not dissolve. In saturated solutes, crystals will form very quickly around the added solute.
To learn more about saturation, refer to the link:
https://brainly.com/question/28215821
#SPJ1
what is the formal charge of a sulfur atom that forms four bonds and has one lone pair?
Answers
In Sulfur Tetrachloride the formal charge of the sulfur atom is zero and in this, it forms four bonds and has one lone pair.
Sulfur tetrachloride, SCl₄, is a fascinating molecule because it exhibits some of the weird concepts in chemical bonding. Sulfur tetrachloride is an inorganic compound with the chemical formula SCl₄. It has simply been obtained as a precarious pale yellow solid. The corresponding SF₄ is a steady, helpful reagent. It melts with concurrent decomposition above -20°C. Its solid configuration is ambivalent. In distinction to this tetrachloride, SF4 is a neutral molecule.
To learn more about sulfur tetrachloride visit here:
https://brainly.com/question/28296216
#SPJ4
Identify the type of reaction being described in Cu(s) + O2(g) = CuO(s)
Answers
6 For each of the following uses, suggest whether you think a pure form of the
metal or an alloy would be better suited. Explain your answer in each case.
Answers
The choice between a pure form of the metal or an alloy depends on the specific use and requirements. Alloys often provide increased strength, durability, and resistance to environmental factors, while pure metals are typically used when electrical conductivity, malleability, or other properties are of utmost importance.
For each use, I will suggest whether a pure form of the metal or an alloy would be better suited.
1. Structural support in buildings - Alloy (e.g., steel)
Alloys like steel provide more strength and durability than pure metals. They are also resistant to corrosion and can handle a higher load.
2. Electrical wiring - Pure form (e.g., copper)
Pure metals like copper have higher electrical conductivity than alloys. This allows for more efficient transmission of electrical currents.
3. Aircraft components - Alloy (e.g., titanium alloy)
Alloys like titanium alloys are lightweight, strong, and resistant to high temperatures, which makes them ideal for aircraft components that need to endure extreme conditions.
4. Jewelry - Both pure form and alloy (e.g., gold and gold alloys)
Pure metals like gold are often used for jewelry because they are malleable, ductile, and aesthetically pleasing. However, alloys are also used to make jewelry more durable and less prone to damage, such as gold alloyed with copper or silver.
5. Kitchen utensils - Alloy (e.g., stainless steel)
Alloys like stainless steel are more resistant to corrosion and wear, making them more suitable for kitchen utensils that are exposed to moisture and high temperatures.
6. Battery components - Pure form (e.g., lithium)
Pure metals like lithium have high energy densities and are efficient in battery applications. The pure form of lithium allows for faster charging and discharging rates.
The choice between a pure form of the metal or an alloy depends on the specific use and requirements. Alloys often provide increased strength, durability, and resistance to environmental factors, while pure metals are typically used when electrical conductivity, malleability, or other properties are of utmost importance.
For more information on alloy kindly visit to
https://brainly.com/question/11353845
#SPJ11
Write a paragraph (5-7 sentences) describing pH and conductivity.
Answers
Answer:
pH is a measure of hydrogen ion concentration, a measure of the acidity or alkalinity of a solution. The pH scale usually ranges from 0 to 14. Aqueous solutions at 25°C with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline. A pH level of 7.0 at 25°C is defined as "neutral" because the concentration of H3O+ equals the concentration of OH− in pure water. On the other hand, electrical conductivity is a non-specific measurement of the concentration of both positively and negatively charged ions within a sample. So the short answer to the question is as follows, the presence of any hydrogen ions present in a substance will impact the pH level and most probably influence conductivity levels. However, hydrogen ions make up only a small part of the ion concentration measured by a conductivity meter.
Provide the condensed formulas for the following (3 points each):a) 1-butanol Blank 1b) ethyl propyl ether Blank 2c) 2,2-dichloropropane Blank 3Blank 1Blank 2Blank 3I
Answers



What are the four scientific goals of the MSL?
Answers
Answer:The fuel/air mixture may become too minimal
Explanation:While cruising at 9,500 feet MSL, the fuel/air mixture is properly adjusted. What will occur if a descent to 4,500 feet MSL is made without readjusting the mixture
the macronutrient most often lacking in in soil to promote optimum plant growth is
Answers
The macronutrient most often lacking in soil to promote optimum plant growth is nitrogen.
Nitrogen is an essential element required for the formation of chlorophyll, which is crucial for photosynthesis in plants. Nitrogen is also required for the synthesis of amino acids, which are the building blocks of proteins. Without adequate nitrogen, plants cannot produce enough proteins, which are necessary for their growth and development.
Nitrogen is naturally present in the soil in the form of organic matter and mineral deposits, but it can become depleted over time due to various factors such as erosion, leaching, and plant uptake. To maintain healthy soil and promote optimum plant growth, it is important to ensure that there is an adequate supply of nitrogen available. This can be achieved through various methods such as adding nitrogen-rich fertilizers, planting nitrogen-fixing crops, and incorporating organic matter into the soil.
It is important to note that while nitrogen is often the most lacking macronutrient in soil, other macronutrients such as phosphorus and potassium can also become depleted and require replenishment for optimal plant growth. Proper soil testing and nutrient management practices can help ensure that plants have access to all the necessary macronutrients for healthy growth and development.
To know more about macronutrient, refer to the link below:
https://brainly.com/question/29789849#
#SPJ11
Can someone please check my answer?

Answers
Yes the ANSWER is Correct-
On first half life the mass will be 10 gram
On second half life the mass will be 5 gram
On third half life the mass will be 2.5 gram