Answers
Related Questions
How many moles of CO2 must dissolve in excess water to produce 12 moles of H2CO3?

Answers
Answer:
12 moles of CO₂.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
CO₂ + H₂O —> H₂CO₃
From the balanced equation above,
1 mole of CO₂ dissolves in water (H₂O) to produce 1 mole of H₂CO₃.
Finally, we shall determine the number of mole of CO₂ needed to produce 12 moles of H₂CO₃. This can be obtained as follow:
From the balanced equation above,
1 mole of CO₂ dissolves in water (H₂O) to produce 1 mole of H₂CO₃.
Therefore, 12 moles of CO₂ will also dissolves in water (H₂O) to produce 12 moles of H₂CO₃.
What did the Constitutional Convention decide to do about the slave trade?(1 point)
Responses
It expanded it.
It expanded it.
It restricted it to slave states.
It restricted it to slave states.
It banned it.
It banned it.
It delayed taking action.
Answers
35.The oxidation numbers in a compound always add up to...Select one:a. 2b. 3c. 1d. 0
Answers
Whenever a compound is formed, it is formed because two or more elements need electrons in order to stabilize and have their 8 electrons, following the octet rule, therefore when we have a compound, the sum of the oxidation numbers must be equal to 0, since we are forming neutral and stable compounds and not ions. Answer letter D
How many molecules are in 3.5 moles of NaCl?
5.8x10-24 molecules
0 203 molecules
O 2.1x1024 molecules
1.5x1024 molecules
Answers
Describe how you would prepare exactly 100 mL of 0.109 M picolinate buffer, pH 5.61. Possible starting materials are pure picolinic acid (pyridine-2-carboxylic acid, FM 123.10), 1.0 M HCl, and 1.0 M NaOH. Place the given steps in order. Not all of the steps will be used.
Answers
Answer:
1.342g of picolinic acid and 6.743mL of 1.0M NaOH diluting the mixture to 100.0mL
Explanation:
The pKa of the picolinic acid is 5.4.
Using Henderson-Hasselbalch formula for picolinic-picolinate buffer:
pH = pKa + log [Picolinate] / [Picolinic]
Where [] could be taken as moles of each species
5.61 = 5.4 + log [Picolinate] / [Picolinic]
0.21 = log [Picolinate] / [Picolinic]
1.62181 = [Picolinate] / [Picolinic] (1)
Now, both picolinate and picolinic acid will be:
0.100L * (0.109mol / L) =
0.0109 moles = [Picolinate] + [Picolinic] (2)
First, as we will start with picolinic acid, we need add:
0.0109 moles picolinic acid * (123.10g/mol) = 1.342g of picolinic acid
Now, replacing (2) in (1):
1.62181 = 0.0109 moles - [Picolinic] / [Picolinic]
1.62181 [Picolinic] = 0.0109 moles - [Picolinic]
2.62181 [Picolinic] = 0.0109 moles
[Picolinic] = 4.157x10⁻³ moles
And:
[Picolinate] = 0.0109 - 4.157x10⁻³ moles =
6.743x10⁻³ molesTo obtain these moles of picolinate ion we need to make the reaction of the picolinic acid with NaOH:
Picolinic acid + NaOH → Picolinate + Water
That means to obtain 6.743x10⁻³ moles of picolinate ion we need to add 6.743x10⁻³ moles of NaOH
6.743x10⁻³ moles of NaOH that is 1.0M are, in mL:
6.743x10⁻³ moles * (1L / 1mol) = 6.743x10⁻³L * 1000 =
6.743mL of the 1.0M NaOH must be addedThus, we obtain the desire moles of picolinate and picolinic acid to obtain the buffer we want, the last step is:
Dilute the mixture to 100mL, the volume we need to prepareFollowing are the calculation to the mass of picolinic acid:
Let pKa of picolinic acid \(= 5.52\)
form the buffer: \(\bold{pH = pKa + \log(\frac{A-}{ HA})}\)
Acid concentration\(= 0.109\)
We will require the conjugate, which would be formed by interaction of picolinic acid and NaOH.
calcultion to the need:
\(pH = pKa + \log(\frac{A-}{ HA})\\\\5.61 = 5.52 + \log(\frac{A-}{ HA})\)
solve for A- by using antilog
\(0.09 = \log(\frac{A-}{ HA})\\\\1.230= \frac{A-}{HA}\)
When
\(HA = 0.109\\\\A- = 0.134\)
If \(V = 100 ml\)then
\(n A- = M\timesV = 0.134 \times 0.1 = 0.0134 \ mol\ of\ A-\)
needed
\(HA + NAOH \to H_2O + Na^+ \ and \ A-\)
therefore,
ratios =1:1
we need 0.0134 mol of NaOH
\(n = M\times V \\\\V = \frac{0.0134}{1} = 0.0134 \ liter \ of\ NaOH\)
but you also want \(0.109 M\) of free picolinic acid so
\(n = M\times V = 0.109\times 0.1 = 0.0109\ mol \ of \ Acid\)
Therefore:
\(n \ acid = 0.0109 + 0.0134\ mol = 0.0243 \ mol\ of \ acid\)
Preparation:
by add volume (19.07) in NaOH
M = 1.0 to the beaker, and by add water until the beaker marks 1 L
Then add 0.0243 mol of picolinic acid
\(\to m = 0.0243 \times 123 = 2.9889\ grams\) of picolinic acid
stir and pH will be that of buffer.
Learn more:
brainly.com/question/17156849
What is the average speed of 50 meters in 3 seconds
Answers
Answer:
16.66 m/s
1. If Steve throws the football 50 meters in 3 seconds, what is the average speed (velocity) of the football? 50m = 16.66 m/s 3s Page 2 2.
Explanation:
The phases of the moon.
Can be predicted years into the future a
Always happen in the same pattern b
Can occur in ANY order c
a and b are correct d
Answers
Answer:
c. or b._____________
Explanation:
correct me
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
In a chemical formula or a chemical equation, what is the small number after the element symbol?
Answers
Answer:
It's called a subscript.
Explanation:
Subscripts tell you how many atoms of an element are in a compound.
It is difficult to measure the volume of a gas
Answers
Answer:
Gases are more difficult to measure than liquids, because measured volumes are highly affected by temperature and pressure. Gas meters measure a defined volume, regardless of the pressurized quantity or quality of the gas flowing through the meter.
Explanation:
It is difficult to measure the volume of agas because measured volumes are highly affected by temperature and pressure.
(B) An organic compound contains C, H, and O. When 1.265g of the compound was burned, 2.417g of carbon dioxide and 1.484g of water were formed. The molecular mass of the compound is 46. Determine its molecular formula? [4]
Answers
Answer:
To determine the molecular formula, we need to find the empirical formula first.
1. Find the moles of CO2 produced:
moles of CO2 = mass of CO2 / molar mass of CO2
moles of CO2 = 2.417g / 44.01 g/mol = 0.05499 mol
2. Find the moles of H2O produced:
moles of H2O = mass of H2O / molar mass of H2O
moles of H2O = 1.484g / 18.02 g/mol = 0.08232 mol
3. Find the moles of C in the compound:
moles of C = moles of CO2
moles of C = 0.05499 mol
4. Find the moles of H in the compound:
moles of H = 2 x moles of H2O
moles of H = 2 x 0.08232 mol = 0.16464 mol
5. Find the moles of O in the compound:
moles of O = (moles of CO2 x 2) + moles of H2O
moles of O = (0.05499 mol x 2) + 0.08232 mol = 0.1923 mol
6. Find the empirical formula:
Divide all the moles by the smallest number of moles to get the simplest whole-number ratio of atoms.
empirical formula = C1H3O1 or CH3O
7. Find the molecular formula:
To find the molecular formula, we need to know the molecular mass of the compound. The molecular mass is 46 g/mol. We can calculate the ratio of the molecular mass to the empirical formula mass:
molecular mass / empirical formula mass = 46 g/mol / 31 g/mol = 1.483
Round this number to the nearest whole number, and multiply the subscripts of the empirical formula by this number to get the molecular formula:
molecular formula = C2H6O2 or C2H6O2
The molecular formula of the compound is C4H4.
To determine the molecular formula of the organic compound, we need to analyze the masses of the elements present and calculate the empirical formula. Given the masses of carbon dioxide (2.417 g) and water (1.484 g) produced during combustion, we can calculate the moles of carbon and hydrogen in the compound.
Moles of carbon:
Using the molar mass of carbon dioxide (44.01 g/mol), we can calculate the moles of carbon dioxide produced:
Moles of carbon dioxide = 2.417 g / 44.01 g/mol = 0.055 moles of CO2
Since there is one mole of carbon in one mole of carbon dioxide, the number of moles of carbon in the compound is also 0.055 moles.
Moles of hydrogen:
Using the molar mass of water (18.015 g/mol), we can calculate the moles of water produced:
Moles of water = 1.484 g / 18.015 g/mol = 0.082 moles of H2O
Since there are two moles of hydrogen in one mole of water, the number of moles of hydrogen in the compound is 2 x 0.082 moles = 0.164 moles.
Now, we can determine the empirical formula by dividing the number of moles of each element by the smallest number of moles (in this case, the number of moles of carbon).
Empirical formula: C0.055H0.055/0.055 = H1
So, the empirical formula of the compound is CH.
To determine the molecular formula, we need to compare the empirical formula mass (CH = 13.02 g/mol) to the given molecular mass (46 g/mol).
Molecular formula: (46 g/mol) / (13.02 g/mol) = 3.53
Since the molecular formula must be a whole number, we round 3.53 to the nearest whole number, which is 4.
For more such questions molecular formula visit:
https://brainly.com/question/26388921
#SPJ8
How are lysosomes and vacuoles the same? How are
they different?
Answers
Answer:
This is the answer they're looking for:
Lysosomes and vacuoles both deal with waste materials. Lysosomes break down waste materials, and vacuoles store waste materials in the cell temporarily before the cell get rids of them.
Explanation:
i hope you'll pick me for brainiest
9. What is the name of the molecule?
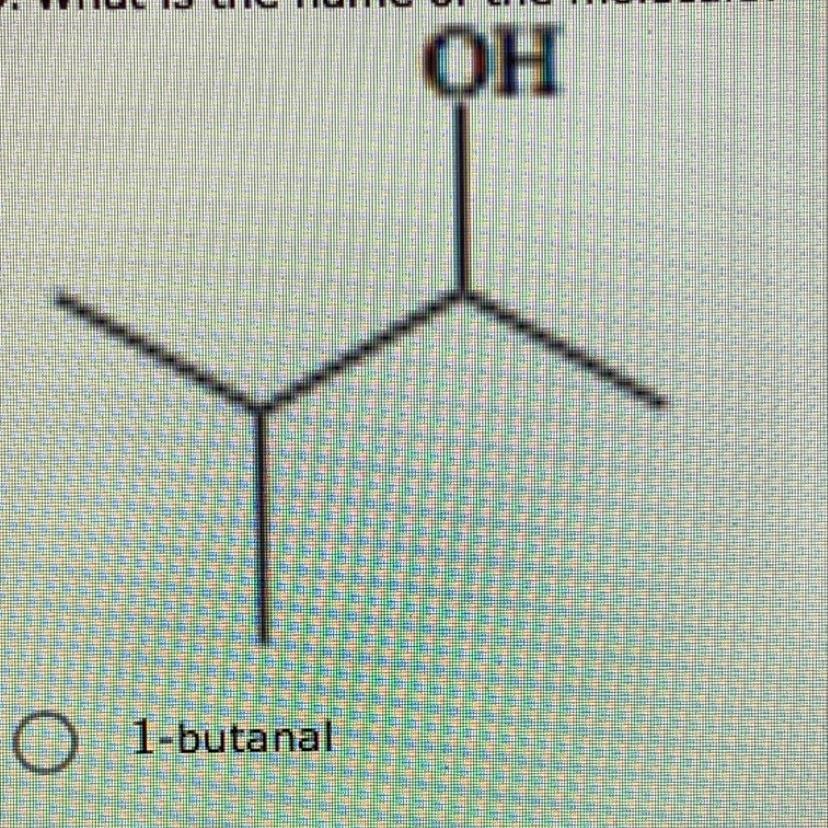
Answers
Answer:
3–methyl–2–butanol
Explanation:
To name the compound, we must:
1. Identify the functional group.
2. Give the functional group of the compound the lowest possible count.
3. Locate the longest continuous carbon chain. This gives the parent name of the compound.
4. Identify the substituent group attached.
5. Give the substituent group the lowest possible count.
6. Combine the above to get the name of the compound.
Now, let us obtain the name of the compound.
1. The functional group of the compound is Alcohol i.e —OH.
2. The functional group is located at carbon 2.
3. The longest continuous carbon chain is carbon 4 i.e butane. But the presence of the functional group i.e OH will replace the –e in butane with –ol. Therefore, the compound is butanol.
4. The substituent group attached is methyl i.e CH3.
5. The substituent group is located at carbon 3.
6. Therefore, the name of the compound is:
3–methyl–2–butanol.
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
what is sodium bicarbonate used for?
Answers
Answer:
Sodium bicarbonate, also known as baking soda, has a wide range of uses in various industries and applications. Here are some of the most common uses of sodium bicarbonate:
Baking: Sodium bicarbonate is widely used in baking as a leavening agent. It reacts with acids, such as cream of tartar, buttermilk, or vinegar, to produce carbon dioxide gas, which causes the dough or batter to rise and become lighter.
Cleaning: Sodium bicarbonate is also used as a natural and effective cleaning agent. It can be used to clean and deodorize carpets, upholstery, kitchen appliances, and bathroom fixtures.
Personal care: Sodium bicarbonate is used in various personal care products, such as toothpaste, deodorants, and bath salts. It is effective in neutralizing odors and as an exfoliant.
Medical: Sodium bicarbonate is used in some medical treatments, such as as an antacid for heartburn or to treat metabolic acidosis, a condition where the body produces too much acid.
Fire extinguisher: Sodium bicarbonate is also used in some fire extinguishers because it can release carbon dioxide gas, which can displace oxygen and extinguish small fires.
Overall, sodium bicarbonate is a versatile substance with many uses in various industries and applications.
PLEASE HELP ASAPPPPPPPPPP

Answers
Answer:
c
Explanation:
Answer:
I think the first one
Explanation:
I think because it is going from 600 to 400 it is decreasing
Find the percentage composition of each compound listed
below. In the first ten problems, the correct formula is given.
In the next four problems, laboratory data for the compound
are presented.
1. FeO
2. MgCl
3. CH4
4. CS₂
5. NO₂
6. HgO
7. SnI₁
8. Cu₂0
9. NH₂
10.CH₂O

Answers
Answer:
Explanation:
% comp is g element / g cpd x 100% get the grams from the periodic table
1) 77.78%Fe & 22.22 % O
2) 40.68 % Mg & 59.32 % Cl
3) 75 % C & 25% H
4) 15.79% C & 84.21 % S
5) 30.43 % N & 69.57 % 0
6) 92.63%Hg & 7.37 % )
7) 18.98 % Sn & 81.02 %I
8) 88.89 % Cu & 11.11 % O
9) 82.35 & N & 17.65 % H
10) 40% C & 6.67% H & 53.33 % O
Consider the reaction 4FeS2 + 11O2 → 2Fe2O3 + 8SO2. If 8 moles of FeS2 react with 15 moles of O2, what is the limiting reactant? (3 points)
SO2
O2
Fe2O3
FeS2
Answers
Answer:
O2
Explanation:
for find the limiting reactant you must calculate the moles of the reactants from the amount that you have and from the MM:
MM FeS2 = 120n = 26.2g / 120g/mol = 0,218 mol
MM O2 = 32n = 5,44g/32g/mol = 0,17 mol
The limiting reactant is
O2
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
The following are steps in an investigation into electrically charged objects.
Step #1: Blow up a balloon.
Step #2: Rub the balloon against your hair.
Step #3: Rub a plastic fork against your hair.
Step #4: Bring the two objects together and observe what happens.
Which steps are required to charge the objects? (2 points)
a
Steps #1 and #2
b
Steps #2 and #3
c
Steps #3 and #4
d
Steps #1 and #4
Answers
b. Steps #2 and #3
Step #1: Blowing up a balloon is not necessary to charge the objects, as it does not involve any rubbing or contact with other materials.
Step #2: Rubbing the balloon against your hair is necessary to charge the balloon.
Step #3: Rubbing a plastic fork against your hair is necessary to charge the fork.
Step #4: Bringing the two objects together and observing what happens is not necessary to charge the objects, but it is necessary to observe the effects of the charges on the objects.
She collected 20 cm³ of oxygen. What volume of hydrogen could she also have collected at the same time?
Answers
Answer:
Assuming it was collected from the atmosphere it would be virtually nothing
Explanation:
hydrogen makes up 0.000055% of the atmosphere while oxygen makes up 23 percent. 20/400000 cm^3 of hydrogen
‼️‼️‼️need help asap‼️‼️‼️

Answers
24. To calculate the molarity of a solution, we must first find out how many moles of \(BaI_2\) are in the solution.
Molar mass of BaI2 = (1 x atomic mass of Ba) + (2 x atomic mass of I)
= (1 x 137.33 g/mol) + (2 x 126.90 g/mol)
= 137.33 g/mol + 253.80 g/mol
= 391.13 g/mol
Number of moles of BaI2 = mass of BaI2 / molar mass of BaI2
= 413 g / 391.13 g/mol
= 1.056 mol
the molarity of the solution using the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
Volume of solution = 750 ml = 750 ml / 1000 ml/L = 0.750 L
Molarity = 1.056 mol / 0.750 L
= 1.408 M
Therefore, the molarity of the solution is 1.408 M.
25. a. \(P_20_7\) - Ionic compound (Phosphorus(V) oxide)
b. \(SnBr_2\) - Ionic compound (Tin(II) bromide)
c. \(Fe(OH)_2\)- Ionic compound (Iron(II) hydroxide)
d. \(Cl_30_8\) - Not a valid chemical formula
26.
A. (NH4)2CO3 is soluble in water (NH4) in an ionic substance called 2CO3 containing the ions carbonate and ammonium.
B. Fe(OH)2 is insoluble in water. Iron(II) hydroxide is only sparingly soluble.
C. CaOH is not soluble in water. Only very little calcium hydroxide is soluble.
D. PbCl2 is insoluble in water. The chloride of lead(II) is sparingly soluble.
27. FeS + 2KCl = FeCl2 + K2S
FeS is an insoluble precipitate.
2KCl dissolves in aqueous solution.
ZnCl2 + SrSO4 = ZnSO4 + SrCl2
SrSO4 is an insoluble precipitate.
ZnCl2 dissolves in aqueous solution.
28. In salt water, the solute is the salt (sodium chloride, or NaCl), and the solvent is water. The element which dissolves in the solvent to form a solution is called solute.
29. Charles's law states that, if the pressure and volume of a gas remain constant, the volume of a gas falls as the temperature increases. As a result, the capacity of the balloon will decrease as it ascends to altitudes where the temperature is -15 °C.
30. The average kinetic energy of the particles of a substance increases with increase in its temperature. This is because temperature is a gauge for the specific kinetic energy of the constituent particles of a substance. On the other hand, the average kinetic energy falls as the temperature increases.
31. When the volume of a gas decreases, its pressure increases. Boyle's law, which states that at a given temperature, the pressure of a gas is inversely proportional to its volume, describes this relationship. On the other hand, pressure falls when volume increases.
32. The pressure of a gas increases along with its temperature. Gay–Lussac's law, which states that the pressure of a gas is directly proportional to its temperature, given the volume and volume of the gas is constant, describes this relationship.
33. The volume of a syringe is reduced as a marshmallow is pressed and the plunger is depressed. As a result the pressure inside the syringe increases. This is because Boyle's law states that the volume and pressure of a gas are inversely proportional. The decrease in volume causes the air inside the syringe to contract, exerting more pressure on the marshmallow, which is then crushed.
Learn more about Charles's law, here:
https://brainly.com/question/12835309
#SPJ1
Most of the elements to the left of the stair-step line in the periodic table exist as _________ in the periodic table.
A. Gases.
B Liquids.
C. Plasmas.
D. Solids.
Answers
Most of the elements to the left of the stair-step line in the periodic table exist as liquids in the periodic table and the correct option is option B.
What is periodic table?
The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their properties.
Elements are arranged from left to right and top to bottom in the order of their increasing atomic numbers. Thus,
Elements in the same group will have the same valence electron configuration and hence, similar chemical properties.Whereas, elements in the same period will have an increasing order of valence electrons. Therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases.Therefore, Most of the elements to the left of the stair-step line in the periodic table exist as liquids in the periodic table and the correct option is option B.
Learn more about Periodic Table, here:
https://brainly.com/question/11155928
#SPJ3
between ethane, ethene and ethyne which is having shortest bond?
Answers
As you can see the picture, in the three given compounds i.e. ethane, ethene and ethyne, ethyne have shortest bond. Bond length of ethyne is very short when compared to the ethane and ethene. Shorter the bond, bond strength will be more. Hence, our answer is ethyne.

Answer:
ethyne has shortest bond
Are you the primary decision maker in your household for automotive-related purchases? yes or no
Answers
Answer:
yes
Explanation:
dont have one
What type of biological molecule is made up of carbon, hydrogen, and oxygenof 1:2:1?A. Lipids3. CarbohydratesC. Nucleic acids. Proteins
Answers
The biological molecule made of only C, H and O with a molar ratio of 1:2:1 is Carbohydrates, and the most commonly known one is glucose, which is the monomer to build large carbohydrate compounds, the chemical formula for glucose is C6H12O6
Which bent shape has more repulsion and a smaller bond angle?
bent 6A
bent 5A
Answers
What is true about dominant alleles? a They almost never appear as the trait. b They appear as the trait only when there are two of them c They appear as the trait over a recessive allele d They appear as the trait if there is not recessive allele
Answers
They appear as the trait over a recessive allele. Statement C) is true about the dominant alleles.
Dominant alleles are genetic variants that, when present in an individual's genotype, are expressed phenotypically, meaning they determine the visible or observable traits. Dominant alleles are represented by capital letters, while recessive alleles are represented by lowercase letters in genetics.
In terms of inheritance, if an individual has at least one copy of the dominant allele, it will be expressed in the phenotype, regardless of the presence of a recessive allele. This is because dominant alleles exert their influence over recessive alleles, thus "dominating" their expression.
To illustrate this, let's consider a specific example using a trait controlled by a single gene with two possible alleles: dominant (A) and recessive (a). If an individual is homozygous dominant (AA), meaning they possess two copies of the dominant allele, the dominant trait will be expressed.
However, if an individual is homozygous recessive (aa), with two copies of the recessive allele, the recessive trait will be expressed since there are no dominant alleles to override it.
Therefore, dominant alleles appear as the trait over recessive alleles, regardless of the presence or absence of a recessive allele. The presence of even a single copy of the dominant allele is sufficient for its expression in the phenotype. Option C
For more such questions on recessive allele visit:
https://brainly.com/question/16048928
#SPJ8
why Mg(OH)2 is soluble in HCL
Answers
Answer:
While Mg(OH)2 is practically insoluble, a certain amount of Mg(OH)2 dissociates into ions when put in water. ... As HCl is added to the beaker containing milk of magnesia, the H+ ions from the HCl react with the OH– ions (those that are actually in solution from the Mg(OH)2) according to Equation 3 below.
