Does gasoline (C8H12) have polar or non-polar molecules?
Answers
Answer:
non polar molecules
Explanation:
Ethyl alcohol will dissolve in water in all proportions because the polar molecules interact with each other freely. In contrast, gasoline is a nonpolar molecule. hope that helped...
Related Questions
what is the most appropriate unit to measure the following; the length of a football field. b) the diameter of a tennis ball
Answers
Explanation:
a) m (metre)
b) mm (millimeter)
An element is
A) made of only one kind of atom.
B) made of compounds from the periodic table.
C) made of different atoms physically combined together.
D) made of different kinds of atoms chemically combined.
Answers
A small can rolled 2 meters in 2 hours.
Answers
what is a property of every mixture
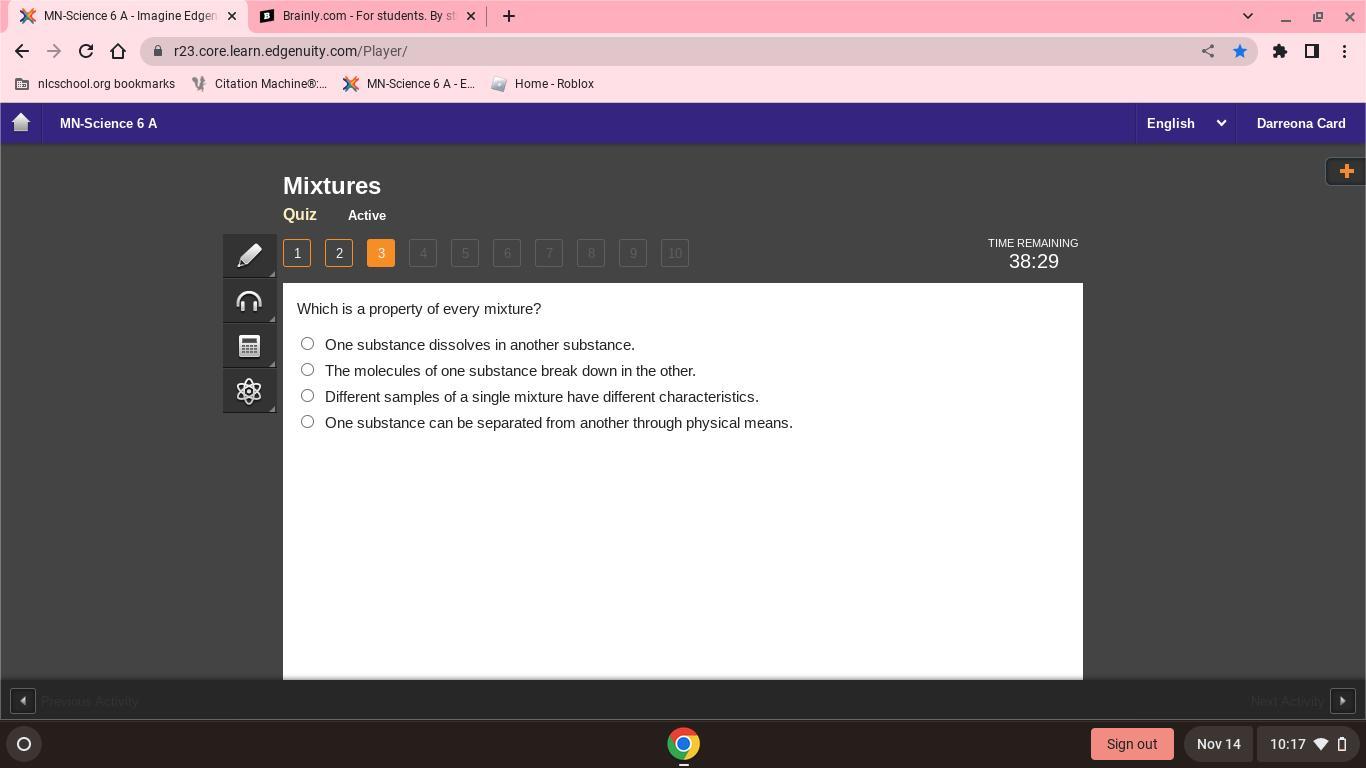
Answers
The property of every mixture is right response is D)One substance can be separated from another through physical means.
A mixture is a combination of two or more substances that are physically merged or mingled without losing their own identities.This indicates that the combination does not alter chemically and can be physically separated (like filtration). It may contain molecules that are solid, liquid, or gaseous.Homogeneous mixtures (having a consistent composition, so that every sample will have the same attribute) and heterogeneous mixtures are the two main categories of mixtures ( non uniform composition that is not every sample will have same property).As a result, D) is a characteristic of every blend.To learn more about mixture visit:
brainly.com/question/24898889
#SPJ1
the molar mass of ccl4 is 153.81 g/mol. how many grams of ccl4 are needed to have 5.000 mol?
Answers
To have 5.000 mol of CCl4, we would need 769.05 grams of it.
To calculate the number of grams of CCl4 needed to have 5.000 mol, we can use the formula:
mass = number of moles x molar mass
Substituting the given values, we get:
mass = 5.000 mol x 153.81 g/mol
mass = 769.05 g
Molar mass is a crucial concept in chemistry as it helps in calculating the amount of substance present in a given sample. The molar mass of any substance is defined as the mass of one mole of that substance. In the case of CCl4, the molar mass is 153.81 g/mol, which means that one mole of CCl4 contains 153.81 grams of the substance.
Using the formula mentioned above, we can calculate the mass of any substance given its number of moles. This is an important calculation as it helps in determining the amount of substance required for a given reaction. In addition, it is also useful in determining the purity of a substance as it can help in comparing the expected mass of a substance to the actual mass obtained.
In conclusion, understanding the concept of molar mass and how to calculate it is essential in chemistry. It helps in determining the amount of substance required for a reaction, analyzing the purity of a substance, and in many other aspects of chemistry.
For more such questions on mol of CCl4
https://brainly.com/question/30187743
#SPJ11
Lab Report: Solubility
Please!! Quick hurry!!
Answers
Answer:
degree to which a substance dissolves in a solvent to make a solution
is this ok
Answer:
Have Fun
Explanation:
Characteristics of an acid and a base
pH scale: What is it? How is it helpful? Where can you find acids and bases on it?
Indicators: Identify two and tell how to use them and what acids and bases look like in them.
Include TWO examples of an acid and a base.
Explain what neutralization is.
Give an example of a neutral substance.
Answers
Answer:
Acids and bases are two types of chemical substances with different characteristics. An acid is a substance that can donate a hydrogen ion, while a base is a substance that can accept a hydrogen ion.
The pH scale is a measurement of how acidic or basic a substance is. It ranges from 0 to 14, with 7 being neutral. Acids have a pH lower than 7, while bases have a pH greater than 7. The pH scale is helpful in identifying the acidity or basicity of a substance, and it can be found in science textbooks or online.
Indicators are substances that change color in the presence of an acid or a base. Two common indicators are litmus paper and phenolphthalein. Litmus paper turns red in the presence of an acid and blue in the presence of a base, while phenolphthalein turns pink in the presence of a base and remains colorless in the presence of an acid.
Examples of acids include lemon juice (citric acid) and vinegar (acetic acid). Examples of bases include baking soda (sodium bicarbonate) and ammonia (NH3).
Neutralization is a chemical reaction between an acid and a base that results in the formation of a neutral substance, such as water and a salt. During neutralization, the acidic and basic properties of the substances cancel each other out, resulting in a neutral solution.
An example of a neutral substance is pure water. When an acid, such as hydrochloric acid (HCl), and a base, such as sodium hydroxide (NaOH), are mixed together in equal amounts, they undergo neutralization to form water and sodium chloride (NaCl).
Why is the C-O bond considered to be polar?
The molecule is symmetrical.
The electronegativity difference of the atoms in the bond.
Answers
Answer:
The electronegativity difference of the atoms in the bond.
Explanation:
Carbon is electropositive while Oxygen is highly electronegative, hence highly polar due to strong intermolecular forces.
The molecule is not symmetrical because it's dipole moments don't cancel out.
I NEED HELP QUICK Activity:
Part 1: Write all bold vocabulary and define the words (See attached PDF File below). Make sure to number each.
Part 2: Look at the "Second Read of Investigating Landforms On Venus" worksheet (Slide 14) and highlight text to the questions on the worksheet.
Part 3: Answer the questions on the "Second Read of Investigating Landforms On Venus" worksheet: 1) How were the novae on Venus similar to the landforms in Geyra's computer model? AND 2) How did the results of Gerya's model provide evidence for what formed the novae on Venus? (Slide 16)
Part 4: Write the notes: (Slide 19)
3) Scientists can use models to test their ideas and get evidence about processes in the natural world that are difficult to observe.
Exit Slip: How do models help scientists answer questions?
Answers
Answer the following questions: A) What species can reduce Sn but not Ni+2? B) What species is the best reducing agent? C) What specie will oxidize Ag E) The oxidation number of sulfur in NazSzOs is F) The oxidation number of non-elemental fluorine is always'
Answers
A) A reducing agent that can reduce Sn but not Ni+2 must have a reduction potential more positive than the reduction potential of Sn2+/Sn (E° = -0.14 V) and less positive than the reduction potential of Ni2+/Ni (E° = -0.23 V).
Therefore, a reducing agent with a reduction potential between these values, such as Fe2+ (E° = -0.44 V), can reduce Sn but not Ni+2.
B) The best reducing agent is the one with the most negative reduction potential. Therefore, among the given reduction the best reducing agent is Li (E° = -3.04 V).
C) A species that can oxidize Ag must have an oxidation potential more positive than the oxidation potential of Ag+/Ag (E° = 0.80 V). Therefore, a species with a higher oxidation potential than this value, such as F2 (E° = 2.87 V), can oxidize Ag.
D) The oxidation number of sulfur in Na2S2O8 is +6.
E) The oxidation number of non-elemental fluorine is always -1, except in some rare compounds where it has a positive oxidation number due to its high electronegativity and tendency to attract electrons.
Learn more about reduction here:
https://brainly.com/question/28813812
#SPJ11
The law of conservation of mass is applicable to
Answers
Answer:
The law of conservation of mass states that in a closed system, mass is neither created nor destroyed during a chemical or physical reaction. The law of conservation of mass is applied whenever you balance a chemical equation.
Explanation:
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
It is applicable in a chemical when the the mass of the products in a chemical reaction is equal to the mass of the reactants.
But it is not applicable in a nuclear fusion as some of the mass is generated as energy.
Using the periodic table, identify the name and symbol of the three neutral atoms given their atomic numbers and masses. The neutral atom with an atomic number of 1 and a mass number of 1. bol. name: Hydrogen atomic symbol: H The neutral atom with an atomic number of 11 and a mass number of 23. name: (Sodium name: Sodium atomic symbol: | 22 Na dionie sympat yang The neutral atom with an atomic number of 7 and a mass number of 14. name: Nitrogen Nitrogen atomic symbol: 0 atomic symbol: N | N º
Answers
The neutral atom with an atomic number of 1 and a mass number of 1 is Hydrogen (H).
The neutral atom with an atomic number of 11 and a mass number of 23 is Sodium (Na).
The neutral atom with an atomic number of 7 and a mass number of 14 is Nitrogen (N).
The atomic number of an element corresponds to the number of protons in its nucleus, which determines its identity. The mass number represents the total number of protons and neutrons in an atom.
For the first atom, with an atomic number of 1 and a mass number of 1, there is only one proton and no neutrons, which corresponds to Hydrogen (H).
The second atom, with an atomic number of 11 and a mass number of 23, has 11 protons and 12 neutrons. This corresponds to the element Sodium (Na).
The third atom, with an atomic number of 7 and a mass number of 14, has 7 protons and 7 neutrons, which corresponds to Nitrogen (N).
To learn more about atomic numbers, here
https://brainly.com/question/16858932
#SPJ4
Explain what causes atoms to bond together to form molecules.
Answers
Atoms contains nucleus in its center and electron that revolve around the atom in fixed orbit. Stability is the reason that causes atoms to bond together to form molecules.
What is atom?Atom is the smallest particle of any element, molecule or compound. Atom can not be further divided. In the nucleus, proton and neutron are present.
Every atoms want to gain stability. To gain stability atoms have to gain noble gas configuration that is outermost shell must contain 2,8, 18 electrons. The atom should follow octet rule. Stability lowers the energy of an atom.
Therefore stability is the reason that causes atoms to bond together to form molecules.
To know more about atom, here:
https://brainly.com/question/13518322
#SPJ1
How many grams are 1.20 x 1025 molecules of calcium iodide?
Answers
Answer:
1230
Explanation:
1.20×1025=1230 is your answer
in some communities forest are cleared through burning in the deforested and is used for farming which of these is negative consequences of this practice
Answers
Explanation:
High emission of CO2 which is bad and the wild animals which live in that spot will flee, thus that place will eventually lose much more ecological diversity.
Using the balanced equation below, how many grams of cesium fluoride would be required to make 73.1 g of cesium xenon heptafluoride?
CSF + XeF6 → CsXeF7
Answers
Answer:
27.9 g
Explanation:
CsF + XeF₆ → CsXeF₇
First we convert 73.1 g of cesium xenon heptafluoride (CsXeF₇) into moles, using its molar mass:
Molar mass of CsXeF₇ = 397.193 g/mol73.1 g CsXeF₇ ÷ 397.193 g/mol = 0.184 mol CsXeF₇As 1 mol of cesium fluoride (CsF) produces 1 mol of CsXeF₇, in order to produce 0.184 moles of CsXeF₇ we would need 0.184 moles of CsF.
Now we convert 0.184 moles of CsF to moles, using the molar mass of CsF:
Molar mass of CsF = 151.9 g/mol0.184 mol * 151.9 g/mol = 27.9 gAl₂(SO4)-2 what is the oxidation number for this

Answers
Here’s a recap: Al is +1
Sulfur: +6
Oxygen: -2
I am a member of the nitrogen family with 16 neutrons.
Answers
Answer:
phosphorus
Explanation:
hope this helps make brainliest and thanks it also 5 stars
have a good day
The element having 16 neutrons in group 15 for the periodic table is phosphorus. Also known as the nitrogen family, group 15 is.
Each of the chemical substances that make up Group 15 (Va) in the periodic table of elements belong to the nitrogen group. Nitrogen (N), phosphorous (P), arsenic (As), arsenic (Sb), bismuth (Bi), plus moscovium (Mc) make up the group. While chemically distinct from one another, the elements exhibit certain basic commonalities in their behaviour, and these similarities are a result of the shared characteristics of their atoms' electronic structures. The element having 16 neutrons in group 15 for the periodic table is phosphorus. Also known as the nitrogen family, group 15 is.
To know more about nitrogen family, here:
https://brainly.com/question/14064572
#SPJ6
in the record of atmospheric co2 concentration at the mauna loa observatory there is a trend and an annual cycle. what is the annual cycle related to?
Answers
The annual cycle is related to the atmospheric CO₂ concentration.
CO₂ is measured as parts per million (ppm), which is numerically equivalent to micromoles of CO₂ per mole of air. The SI unit for CO2 concentration is µmol per mol. CO₂ typically decreases during sunny, warm days because of photosynthesis removes CO₂ from the air. This generally occurs in summer days.
In the record of atmospheric CO₂ concentration at the Mauna loa observatory there is a trend and an annual cycle. The trend of the Mauna loa observatory with atmospheric CO₂ concentration is in the upward trend.
Learn more about atmospheric CO₂ concentration.
https://brainly.com/question/28428799
#SPJ4
Find the Break-Even point
Fixed costs = $790
Revenues (selling price x # sold) = $1. 75 x 180
Variable costs = $81
# of units = 346
Answers
To find the break-even point, we need to determine the number of units at which the total cost equals the total revenue. In this case, variable costs of $81, the break-even point is at approximately 459 units.
The break-even point is the point at which the total cost equals the total revenue, resulting in zero profit or loss. To calculate the break-even point, we need to consider the fixed costs, variable costs, and revenues.
In this scenario, the fixed costs are given as $790, the revenues are $1.75 per unit for 180 units sold, and the variable costs are $81. The formula for calculating the break-even point is:
Break-even point = Fixed costs / (Selling price per unit - Variable cost per unit)
Substituting the given values, we have:
Break-even point = $790 / ($1.75 - $81/180)
Simplifying the equation, we find:
Break-even point ≈ 459 units
Therefore, the break-even point is approximately 459 units, meaning that the company needs to sell at least 459 units to cover its costs and avoid a loss.
Learn more about break-even point here
https://brainly.com/question/24610608
#SPJ11
How many moles of copper would be needed to make one mole of cu2o?
Answers
Answer:
Explanation:
You can view more details on each measurement unit: molecular weight of Copper(I) Oxide or grams The molecular formula for Copper(I) Oxide is Cu2O. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Copper(I) Oxide, or 143.0914 grams.
To balance the reaction what coefficients (numbers) are needed: HBr +KOH ---> KBr + H2O
Answers
Answer:
H2Br + 2KOH ----- K2Br + 2H2O
_____ is the first enzyme to synthesize a nucleic acid at a replication fork.
Answers
The first enzyme to synthesize a nucleic acid at a replication fork is DNA polymerase. This enzyme plays a crucial role in the process of DNA replication, ensuring accurate copying of genetic information.
Here is a step-by-step explanation of its role:
1. DNA replication begins at a specific location on the DNA molecule called the origin of replication.
2. The two strands of the DNA double helix are separated by the action of a helicase enzyme, creating a replication fork with single-stranded DNA on both sides.
3. Single-strand binding proteins stabilize the single-stranded DNA at the replication fork.
4. The enzyme primase synthesizes short RNA primers, providing a starting point for DNA polymerase to begin synthesis.
5. DNA polymerase adds nucleotides to the 3' end of the RNA primer, synthesizing the new DNA strand in the 5' to 3' direction.
6. As the replication fork moves along the DNA, DNA polymerase continues to add nucleotides, building the new strand complementary to the template strand.
7. In the lagging strand, DNA replication occurs discontinuously, creating Okazaki fragments that are later joined by DNA ligase.
In summary, DNA polymerase is the first enzyme to synthesize a nucleic acid at a replication fork, playing a vital role in accurately replicating genetic information within cells.
Learn more about DNA polymerase here: brainly.com/question/14315652
#SPJ11
in what way are the electron configurations of h, li, na, k, rb, and cs similar?
Answers
Answer:
These elements are of s-block and the all have
ns¹ (n = 1 to 7) electronic configuration
I would like to know if question number 3 is rite

Answers
The balanced reaction of this chemical reaction is as follows :
\(2HClO_4(aq)\text{ + Ba}(OH)_2(aq)\text{ }\Rightarrow Ba(ClO_4)_2(aq)+2H_2O(l)\text{ }\)• As we can see, this is a double-displament reaction
what happens to the gaseous phase of water when it reaches a metal lid
Answers
Answer:
When the gaseous phase of water reaches a metal lid it condenses to form water.
Explanation:
The gaseous phase of water is at a higher temperature than the temperature of a metal lid. Thus when it comes in contact with the cold lid it loses heat and gets converted to the liquid phase.
When water vapor loses temperature, it undergoes a process called condensation, which results in its conversion to a liquid state. This process is essentially the opposite of vaporization. Typically, condensation occurs when a vapor is compressed or cooled to its saturation limit, causing the molecular density within the gas phase to reach its maximum threshold.
To know more about condensation,
https://brainly.com/question/30629848
A very large tank initially contains 100 L of pure water. Starting at time t=0 a solution with a salt concentration of 0.7 kg/L is added at a rate of 6 L/min. The solution is kept thoroughly mixed and is drained from the tank at a rate of 4 L/min. Answer the following questions. 1. Let y(t) be the amount of salt (in kilograms) in the tank after t minutes. What differential equation does y satisfy? Use the variable y for y(t). Answer (in kilograms per minute):
dt
dy
= 2. How much salt is in the tank after 30 minutes? Answer (in kilograms):
Answers
After 30 minutes, there would be 1.05 kilograms of salt in the tank.
1. The differential equation that y(t) satisfies can be obtained by considering the rate of change of salt in the tank. The rate at which salt is added to the tank is given by the concentration of the solution (0.7 kg/L) multiplied by the rate at which the solution is added (6 L/min). The rate at which salt is drained from the tank is given by the concentration of salt in the tank (y(t) kg/L) multiplied by the rate at which the solution is drained (4 L/min). Therefore, the differential equation is:
dy/dt = (0.7 kg/L * 6 L/min) - (y(t) kg/L * 4 L/min)
Simplifying further, we have:
dy/dt = 4.2 - 4y(t)
2. To determine the amount of salt in the tank after 30 minutes, we need to solve the differential equation. One approach is to find the particular solution by assuming y(t) takes the form of a constant, y. Substituting this into the differential equation, we have:
dy/dt = 4.2 - 4y
Setting dy/dt to zero (since y is constant), we can solve for y:
0 = 4.2 - 4y
4y = 4.2
y = 4.2/4
y = 1.05 kg
Therefore, after 30 minutes, there would be 1.05 kilograms of salt in the tank.
Learn more about Constant https://brainly.com/question/27983400
#SPJ11
when h2co3 dissociates into h and hco3- does the ph of the water increase or decrease?
Answers
When H2CO3 dissociates into H and HCO3- the pH of water increases to an extent but not much.
The dissociation of H2CO3 is as follows;H2CO3-->H+ + HCO3-
H2CO3 is considered an acid because it ionizes in H+ and HCO3-. Some of the HCO3- will bond with water and form OH- and H2CO3 again. This is an equilibrium. But If we have only one HCO3- that didn't yet bond to water and formed H2CO3 again, we will have more H+ in the medium, and it will be acid. In fact, there is a constant called Ka that measures how much HCO3- and H2CO3 are in a solution in a given time.The concentration of H+ ions doesn't increase much on dissociation so pH doesn't increase much but to an extent.To learn more about pH visit:
brainly.com/question/491373
#SPJ4
When 0.103 g of Zn( s ) is combined with enough HCl to make 50.0 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.5 C to 23.7 C. Find Hrxn for this reaction as written. (Use 1.0 g/mL for the density of the solution and 4.18 J/g
Answers
The answer is -0.251 kJ as Hrxn for this reaction as written.
The reaction between Zn( s ) and HCl can be described as follows:Zn(s) + 2HCl(aq) ⟶ ZnCl2(aq) + H2(g)From the balanced equation above, one mole of zinc produces one mole of hydrogen gas. Hence, 0.103 g of zinc produced:0.103g Zn(1mol/65.38g) = 1.579 × 10-3 moles of Zn.Moles of H2 = 1.579 × 10-3 moles by stoichiometry.Mass of solution = 50.0 g/1.0 mL × 1.0 g/mL = 50.0 g.Let's calculate the change in temperature of the solution:ΔT = Tfinal – Tinitial = 23.7 °C – 22.5 °C = 1.2 °C.From the above information, we can calculate the heat of reaction as follows:qrxn = -(Cp × m × ΔT)where Cp = heat capacity of the solution, m = mass of solution, and ΔT = change in temperature of the solution.
The heat capacity of the solution, Cp = 4.18 J/g °C.Mass of solution, m = 50.0 g.So, qrxn = -(4.18 J/g °C × 50.0 g × 1.2 °C) = -251 J.Hence, the value of Hrxn is -251 J or -0.251 kJ. Therefore, the answer is -0.251 kJ as Hrxn for this reaction as written.
Learn more about Hydrogen here,What are the uses of hydrogen?
https://brainly.com/question/24433860
#SPJ11
Which of the following best explains the distinctive colors of specific elements when used in
fireworks?
A
the number of electrons in the atoms
B
electrons moving from an excited energy state to the ground state
С
the number of neutrons in the atoms
D
electrons transferring from one atom to a different atom
Answers
The distinctive colors of specific elements when used in fireworks owes to electrons moving from an excited energy state to the ground state.
Neils Bohr in his atomic model postulated that electrons in atoms are found in energy levels. Each energy level corresponds to a fixed amount of energy.
The lowest energy level in the atom is called the ground state and higher energy levels are called excited states.
When an electron receives energy, it can move from a ground state to excited state.
This excess energy is given out as visible light of a characteristic wavelength or color when the electron is moving from an excited energy state to the ground state.
This accounts for the distinctive colors of specific elements when used in
fireworks.
Learn more: https://brainly.com/question/1596638