Answers
The Lewis structure of the compound\(CH_{3} Br\) is shown in the image attached.
What is the Lewis structure?The Lewis structure of a molecule or ion is produced by arranging the atoms in a manner that lessens the attraction between their valence electron pairs and then distributes the valence electrons among the atoms to form covalent bonds.
The octet rule, which states that atoms normally gain or lose electrons to obtain a stable configuration with eight valence electrons, frequently serves as a guidance when arranging electrons in the Lewis structure.
Learn more about Lewis structure:https://brainly.com/question/20300458
#SPJ1

Related Questions
What is the answer to this question?

Answers
Answer:
Its is C urea if this is correct please make me brainliest
2. Which state of matter is characterized by particles that are close to each other but are not arranged in a definite pattern?
A)liquid
B)plasma
C)solid
D)gas
Answers
Answer:
Solid
Explanation:
Cus its solid, take a brick for example. It's hard and has no space unlike liquid or gas.
Which of the following is a hormone produced by the posterior pituitary?a. oxytocinb. ADHc. prolactind. None of the listed responses is correct.
Answers
The hormone that produced by the posterior pituitary is none of the listed responses is correct (D)
The posterior pituitary is one of the two lobes that form the pituitary gland, which is a little endocrine gland about the size of a pea and located at the base of the brain. The primary functions of the posterior pituitary include the storage and release of the hormone oxytocin and antidiuretic hormone (ADH, or vasopressin). The anterior lobe and the posterior lobe are the two components that make up the pituitary glands.
The anterior lobe is responsible for the production and release of hormones. It is the nerve cells in the hypothalamus that are responsible for the production of hormones; nevertheless, the posterior lobe is responsible for their release into the circulation of the body.
Thus, there is none of the listed responses is correct since the one that responsible for the production of hormones is hypothalamus.
To learn more about the posterior pituitary, click here:
https://brainly.com/question/23035588
#SPJ4
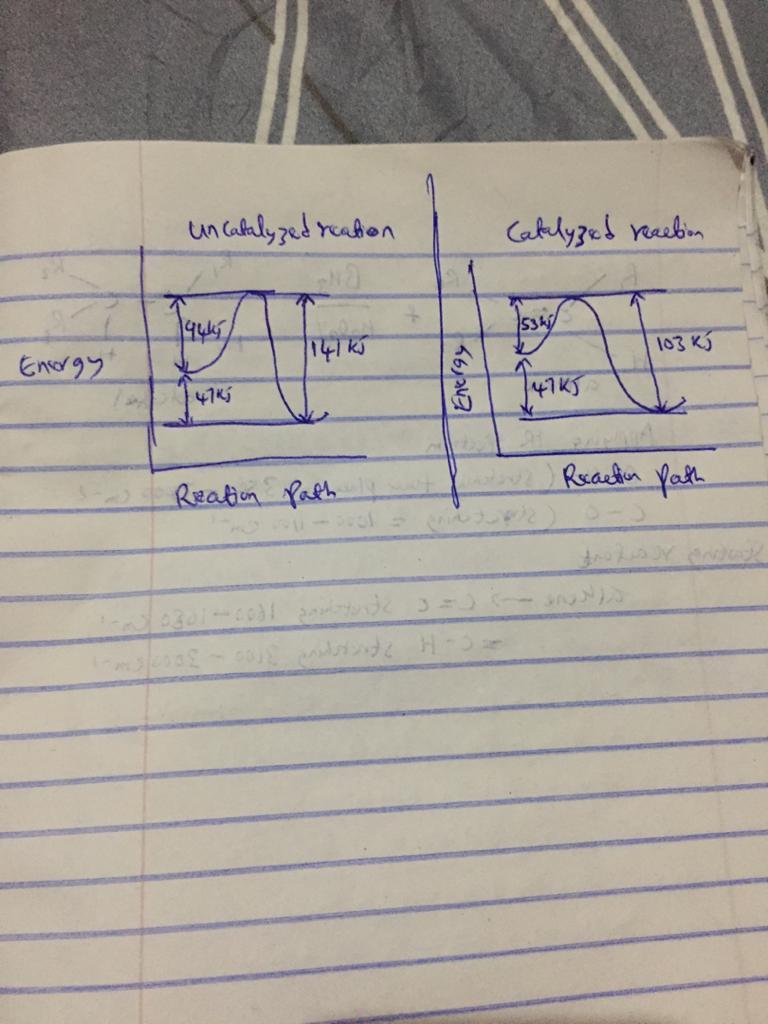
A catalyst decreases the activation energy of a particular exothermic reaction by 56 kJ/mol, to 35 kJ/mol. Assuming that the mechanism has only one step, and that the products are 78 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions. What is the activation energy for the uncatalyzed reverse reaction
Answers
Answer:
Activation energy for the uncatalyzed reverse reaction = 103 kJ/mol
Explanation:
Activation energy decreases from = 56 kj/mol to 35 kj/mol
products = 78 KJ lower in energy than reactants
Activation energy for the uncatalyzed reverse reaction = 103 kJ/mol
attached below are the sketches of approximate energy-level for both catalyzed and uncatalyzed reactions
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Which of the following scenarios best describes separation by distillation?
A sample of ocean water is boiled in a glass flask. A solid remains in the flask, and a hot gas flows
into a cooling chamber. When the as cools enough, it condenses back into a liquid in a separate flask.
O a stip of paper with a sample of ink is placed in a jar. The liquid runs up the paper, resulting in
colored bars running up in a line.
O A funnel and porous paper are used to separate solids in a sample of dirty water from the liquid.
O Aliquid sample is spun quickly in a centrifuge. A layer of solid forms on the bottom of the test tube
over time.
Answers
into a cooling chamber. When the gas cools enough, it condenses back into a liquid in a separate flask.
If the ΔH = 144 kJ/mol and ΔS = 54 J/K mol for a nonspontaneous reaction, at what temperature does this reaction become spontaneous?
Answers
The reaction becomes spontaneous at approximately 2667 Kelvin.
To determine the temperature at which a nonspontaneous reaction becomes spontaneous, we can use the equation ΔG = ΔH - TΔS, where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
In this case, we are given ΔH = 144 kJ/mol and ΔS = 54 J/K mol. To convert ΔH to J/mol, we multiply by 1000, giving us ΔH = 144,000 J/mol.
To find the temperature at which the reaction becomes spontaneous, we set ΔG to zero, as this is the condition for equilibrium. Thus, we have 0 = ΔH - TΔS.
Rearranging the equation, we have TΔS = ΔH, and substituting the given values, we get T * 54 J/K mol = 144,000 J/mol.
Dividing both sides by 54 J/K mol, we find that T = 144,000 J/mol / 54 J/K mol = 2667 K.
Therefore, the reaction becomes spontaneous at approximately 2667 Kelvin.
For more question on reaction
https://brainly.com/question/25769000
#SPJ8
Which of the following does NOT demonstrate the law of conservation of matter?
check all that apply

Answers
The one that does not demonstrates the law of conservation of matter would be the third option: \(2NO_2 + H_2O -- > HNO_3 + HNO_2\)
What is law of conservation of matter?It is a law that explains that matters are conserved during the course of chemical reactions. They can neither be destroyed nor created.
Thus, the number of moles of the species in a reaction remains the same before and after the reaction.
Thus, an equation that demonstrates the law of conservation of matter will be balanced in terms of the number of moles of species.
The only equation, in this case, is \(2NO_2 + H_2O -- > HNO_3 + HNO_2\)
There are 3 oxygen species on the reactant while 5 are present on the product side.
More on the law of conservation of matter can be found here: https://brainly.com/question/9434062
#SPJ1
How many joules are required to increase the temperature of 30.0 g of water from 10⁰ C to 100⁰ C vapors.
Answers
Answer
11302.2 joules
Explanation
Given:
Mass of water = 30.0 g
Initial temperature, T₁ = 10⁰ C
Final temperature, T₂ = 100⁰ C
Increase in temperature, ΔT = T₂ - T₁ = 100⁰ C - 10⁰ C = 90⁰ C
What to find:
The energy in joules required to increase the temperature of the water.
Step-by-step solution:
The joules required to increase the temperature of the water can be calculated using the formula:
q = mcΔT
Where q is the joules of energy required.
m is the mass of water = 30.0 g
c is the specific heat of water = 4.186 J/g ⁰C
ΔT is the temperature rise = 90 ⁰C
∴ q = 30.0 g x 4.186 J/g ⁰C x 90 ⁰C
q = 11302.2 joules
Hence, 11302.2 joules are required to increase the temperature of 30.0 g of water from 10⁰ C to 100⁰ C vapors.
100 POINTS!!!
What is the average rate of the reaction over the entire course of the reaction?
1.6 × 10−3 (?)
1.9 × 10−3 (?)
2.0 × 10−3 (X)
2.2 × 10−3 (X)

Answers
Answer:
b. 1.9 × 10-3
Explanation:
Answer:1.9x10-3
Explanation:
average
It can take hundreds of billions of years for a red dwarf to convert all of its hydrogen completely to helium.
a. True
b. False
Answers
It can take hundreds of billions of years for a red dwarf to convert all of its hydrogen completely to helium is false it takes less than 14 billion years to completely burn through its fuel.
What does red dwarf star mean?A red dwarf is one of the smallest and coldest stars on the main sequence. As red people are the most common type of star in the Milky Way, at least in the vicinity of the Sun, but because of their low luminosity, people cannot be easily observed.
In the solar core, the temperature and density are gigantic and cause nuclear fusion of hydrogen into helium. Four hydrogen atoms fuse to form a helium atom. And this fusion is an incredible way to produce energy.
See more about red dwarf at brainly.com/question/25981690
What is the pH of a solution of 0.300 mole of acetic acid (CH3CO₂H) (Ka = 1.8 × 10-5) and 1.50
moles of sodium acetate (NaCH3CO₂) dissolved in 1.00 L of water?
Answers
The pH of the solution is 2.44.
we first need to write the chemical equation for the dissociation of acetic acid in water:
\(CH_3CO_2H + H_2O - > CH_3CO_2^- + H_3O^+\)
We can use the acid dissociation constant (Ka) for acetic acid to determine the concentration of hydronium ions (H3O+) in the solution, and then use the pH equation to calculate the pH:
Ka = [CH₃CO₂⁻][H₃O⁺] / [CH₃CO₂H]
We know the concentrations of acetic acid and sodium acetate, so we can calculate the concentration of acetate ions ([CH₃CO₂⁻]) using the stoichiometry of the reaction:
[CH₃CO₂⁻] = [NaCH₃CO₂] = 1.50 moles / 1.00 L = 1.50 M
We can assume that all of the acetic acids dissociate into acetate ions and hydronium ions, so the initial concentration of acetic acid is equal to the change in concentration of acetate ions:
[CH₃CO₂H] = 0.300 moles / 1.00 L = 0.300 M
[CH₃CO₂⁻] = [H3O+] = x
Substituting these values into the Ka expression, we get:
1.8 x 10⁻⁵ = (1.50 M)(x) / (0.300 M)
Solving for x, we get:
x = 0.0036 M
Now, we can use the pH equation to calculate the pH:
pH = -log[H₃O+]
pH = -log(0.0036)
pH = 2.44
Therefore, the pH of the solution is 2.44.
learn more about pH here
https://brainly.com/question/172153
#SPJ9
A procedure involving removal of the coronal portion of an exposed vital pulp is a
Answers
Answer:
ExplanaPulpotomy is a vital pulp therapy in which the coronal portion of the pulp is removed surgically and the remaining radicular pulp is preserved intact. Over the remaining radicular pulp tissue, a suitable material is placed which has the potential to protect the pulp from further insult and initiate healing and repairtion:
Answer:
preserve of the vitality of the remaining portion of the pulp within the root of the tooth. Also referred to as root canal therapy
Explanation:
(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
Why does CO2 have a higher boiling point than CH4 when they both possess dispersion forces?
Answers
CO₂ have a higher boiling point than CH₄ when they both possess dispersion forces because CO₂ consists of polar bonds between carbon and oxygen.
Intermolecular forces determine bulk properties, such as the melting points of solids and the boiling points of liquids.
Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid.
CO₂ have a higher boiling point than CH₄ when they both possess dispersion forces because CO₂ consists of polar bonds between carbon and oxygen. The polarity increases the boiling point of the molecule.
Learn more about Intermolecular forces, here:
https://brainly.com/question/31797315
#SPJ1
please help me! ASAP

Answers
Answer:
(1) 4- ethyl -6 methyl -2 octyne
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
A block of metal has a mass of 18.361kg and the following dimensions: 22.35 cm x 7.34 cm x 22.05 mm. What is the density of the metal in g/cm^3
Answers
Answer:
50.76 g/cm³
Explanation:
We'll begin by converting 18.361 Kg to g. This can be obtained as follow:
1 Kg = 1000 g
Therefore,
18.361 Kg = 18.361 Kg × 1000 g / 1 Kg
18.361 Kg = 18361 g
Next, we shall express the dimension in the same unit of measurement.
Dimension = 22.35 cm x 7.34 cm x 22.05 mm
Thus, we shall convert 22.05 mm to cm. This can be obtained as follow:
10 mm = 1 cm
Therefore,
22.05 mm = 22.05 mm × 1 cm / 10 mm
22.05 mm = 2.205 cm
Thus, the dimension is 22.35 cm x 7.34 cm x 2.205 cm.
Next, we shall determine the volume of the metal. This can be obtained as follow:
Dimension = 22.35 cm x 7.34 cm x 2.205 cm.
Volume = 361.728045 cm³
Finally, we shall determine the density of the metal. This can be obtained as follow:
Mass of metal = 18361 g
Volume of metal = 361.728045 cm³
Density of metal =?
Density = mass / volume
Density of metal = 18361 / 361.728045
Density of metal = 50.76 g/cm³
A cylinder of argon gas contains 50.0 L or Ar at 18.4 atm and 'C. How many moles of argon are in the cylinder?
Answers
A cylinder of argon gas contains 50.0 L of Ar at 18.4 atm and 127 °C. How many moles of argon are in the cylinder?
Number moles of Argon : 28.03
Further explanationGiven
The volume of gas=50 L
P = 18.4 atm
T = 127+273=400 K
Required
moles of Argon
Solution
Use ideal gas Law :
\(\tt n=\dfrac{PV}{RT}\\\\n=\dfrac{18.4\times 50}{0.08205\times 400}\\\\n=28.03\)
Calculate the mass of NaCl required to prepare 360.0 mL of a 0.35 M solution.
Answers
Answer:
Explanation:
Molar mass of NaCl = 58.44
Molar mass of NaCl(58.44g) addding to 1000ml give rise to 1M solution always, this can be writtne as below
58.44----- >1000 ml ---> 1M
X gram ----360 ml ---0.35 M
X Gram= 58.44*360*0.35/1000*1 = 7.3 gram
hope you understood the solution
1. Based on the observed performance of the air bag models and the amounts of sodium bicarbonate and acetic acid (vinegar) needed for an automotive air bag of 80 or 160 L, are these reactants a good substitute for sodium azide? One additional note regarding sodium azide: the rate of inflation after a triggering impact is 40 milliseconds (0.04 s).
Answers
Sodium bicarbonate and acetic acid are not good substitute for sodium azide in airbags since the require more mass and produce less gas.
Which is the better chemical for an airbag?The chemical equation for the production of nitrogen gas from sodium azide is given below:
NaN₃ → 2 Na + 3 N₂1 mole or 66 go of sodium azide produces 3 moles or 67.2 L of nitrogen gas.
The equation for the production of carbon dioxide from sodium bicarbonate and acetic acid is given below:
Na₂CO₃ + CH₃COOH → CH₃COONa + CO₂ + H₂O1 mole, 106 g of Na₂CO₃ and 1 mole, 82 g of CH₃COOH are required to produce 1 mole or 22.4 L of CO₂.
The mass of sodium azide required is less than that of sodium bicarbonate and acetic acid required. Also, sodium azide produces a greater volume of gas. Therefore, sodium bicarbonate and acetic acid are not good substitute for sodium azide in airbags.
In conclusion, sodium azide is a better choice in airbags.
Learn more about airbags at: https://brainly.com/question/14954949
#SPJ1
what are the products obtained from petroleum?
Answers
Petroleum is a naturally occurring liquid mixture of hydrocarbons, which is usually referred to as crude oil. It is a non-renewable resource that is extracted from the ground by drilling wells.
Petroleum is a complex mixture of various components, and it is refined into different products for use in different industries. There are various products obtained from petroleum. These products include gasoline, diesel fuel, heating oil, jet fuel, kerosene, asphalt, lubricants, and petrochemicals. Each of these products has its own unique properties and uses.
1. Gasoline: Gasoline is the most commonly used petroleum product. It is a liquid fuel that is used in internal combustion engines in cars, trucks, and other vehicles. Gasoline is a mixture of various hydrocarbons that have been refined from crude oil.
2. Diesel Fuel : Diesel fuel is another liquid fuel that is obtained from petroleum. It is used in diesel engines in trucks, buses, and other heavy-duty vehicles. Diesel fuel is made up of hydrocarbons that are heavier than those in gasoline.
3. Heating Oil :Heating oil is a liquid fuel that is used to heat homes and buildings. It is similar to diesel fuel but is refined to have a higher boiling point.
4. Jet Fuel: Jet fuel is a type of kerosene that is used to power jet engines in airplanes. It is refined to have a low freezing point and a high energy content.
5. Kerosene: Kerosene is a liquid fuel that is used for lighting, heating, and cooking. It is similar to jet fuel but is refined to have a higher boiling point.
6. Lubricants: Lubricants are oils that are used to reduce friction between moving parts in engines and machinery. They are made from refined petroleum and can be used in a variety of applications.
7. Petrochemicals: Petrochemicals are chemicals that are derived from petroleum. They are used in a wide range of products, including plastics, synthetic fibers, rubber, and detergents.
Overall, petroleum is an important resource that is used to produce a wide range of products that we use in our daily lives. The products obtained from petroleum have a significant impact on the economy, transportation, and various industries.
Know more about Lubricants here:
https://brainly.com/question/20427687
#SPJ8
when 12.0 g of hydrogen reacts with oxygen, how many grams of water are produced?
Answers
Answer:
12.0 grams of hydrogen would produce 12.0 grams of water.
Explanation:
The reaction between hydrogen and oxygen to form water can be represented as follows:
2H2(g) + O2(g) -> 2H2O(l)
From the equation, it can be seen that for every 2 grams of hydrogen, 1 gram of oxygen is required to produce 2 grams of water. Therefore, if 12.0 grams of hydrogen reacts with oxygen, 12.0/2 = 6.0 grams of oxygen would be required. This amount of oxygen would produce 2 * 6.0 = 12.0 grams of water.
Answer:
Explanation:
the grams of water produced= 44 grames
If atmospheric pressure on a certain day is 743 mmHg. what is the partial pressure of nitrogen, given that nitrogen makes up about 78% of atmospheric air?

Answers
Answer
The partial pressure of nitrogen = 579.54 mmHg.
Explanation
Given that nitrogen makes up about 78 % of atmospheric air.
Mole fraction of nitrogen in the atmosphere = 78/100 = 0.78
Total pressure of the air = 743 mmHg
So according to Raoult's law:
\(\begin{gathered} P_{N_2}=\chi_{_{N_2}}\times P_{total} \\ \\ Put\text{ }\chi_{_{N_2}}=0.78,\text{ }P_{total}=743\text{ }mmHg \\ \\ P_{N_2}=0.78\times743\text{ }mmHg=579.54\text{ }mmHg \end{gathered}\)The partial pressure of nitrogen = 579.54 mmHg.
what causes the different crusts to rise and lower?
Answers
Answer:
tectonic plates
Explanation:
What is the Internal Energy of a Human Body? Define internal energy as Delta E.
SHOW ALL WORK!
20 points
Answers
The internal energy of a human body is the net energy contained in the body due motion of its particle or molecules.
In a human body the internal energy is stored . It increases when the temperature of the body rises, or when the body observes some changes. Internal energy we can say that is the sum of kinetic and potential energy of all particles in the body.
Internal energy is a state function of a system and is an extensive quantity. Every substance possesses a fixed quantity of energy which depends upon its chemical nature and also on its state of existence. Every substance have a definite value of Internal energy.
The change in the Internal energy of a reaction may be considered as the difference between the internal energies of the two states.
ΔU = \(E_{B}\) - \(E_{A}\) where \(E_{B}\) and \(E_{A}\) are the initial energies of states A and B. Or we can also write the equation as:
ΔU = ΔE
To know more about Internal energy
https://brainly.com/question/13125947
#SPJ1
There is 45% oxygen, 33% sodium and 22% sulfur. What is the empirical formula?
Answers
Hello
To solve this question, let's change the percentages into grams.
\(\begin{gathered} O=45g \\ Na=33g \\ S=22g \end{gathered}\)Step 2
convert the grams into moles
but the formula of mole is given as
\(n=\frac{\text{mass}}{\text{molar mass}}\)Let's do this
\(\begin{gathered} O=\frac{45}{16}=2.8125moles \\ Na=\frac{33}{23}=1.434moles \\ S=\frac{22}{32}=0.6875\text{moles} \end{gathered}\)Lastly, we take the ratio of the atoms
\(\begin{gathered} \frac{O}{S}=\frac{2.8125}{0.6875}=4.09 \\ \frac{Na}{S}=\frac{1.434}{0.6875}=2.085 \end{gathered}\)From the calculations above, using the ratio of the atoms, the emperical formula of the compound is Na₂SO₄
Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is given below.
Fe2O3(s) + 2 Al(s) 2 Fe(l) + Al2O3(s)
What masses of iron(III) oxide and aluminum must be used to produce 30.0 g iron?
What is the maximum mass of aluminum oxide that could be produced?
Answers
a) The mass of the iron III oxide is 43.2 g
b) The mass of aluminum oxide is 13.77 g
What is the mass that is used?We would need to look at the stoichiometry of the reaction as this would help us to begin to understand how to deal with the question that is at hand here.
Number of moles of the iron = 30.0 g/56 = 0.54 moles
If 1 mole of the iron III oxide produces 2 moles of iron
x moles of the iron III oxide would produce 0.54 moles
x = 0.27 moles
Mass of the iron III oxide = 0.27 moles * 160 g/mol
= 43.2 g
Given that the ratio of the iron and the aluminum oxide is 1:1
1 mole of aluminum oxide is obtained for every 2 moles of iron
x moles of the aluminum oxide is produced for 0.27 moles
x = 0.135 moles
Mass of the aluminum oxide = 0.135 moles * 102 g/mol
= 13.77 g
Learn more about stoichiometry:https://brainly.com/question/30215297
#SPJ1
"write the chemical equation between hydrogen (h) and carbonate (CO3) and the product(s)"
Answers
Answer:
On heating sodium hydrogen carbonate (NaHCO3). It decomposes to form sodium carbonate (Na2CO3), water (H2O) and carbon dioxide (CO2)
(ii) 2NaHCO3(s)Heatsodiumcarbonate(sodaash)Na2CO3(s)+CO2(g)+H2O(l)
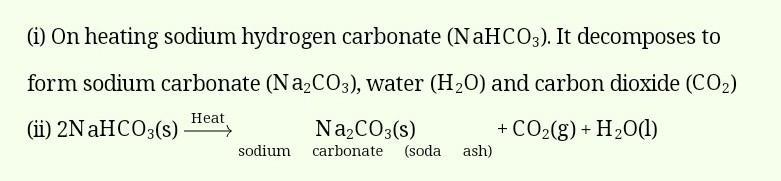
Answer:
Explanation:
2H + + CO3 2- = H2CO3
Good evening everyone Write any three difference between symbol and molecular formula answer it ASAP thank u
Answers
Answer:
A molecular formula consists of the chemical symbols for the constituent elements followed by numeric subscripts describing the number of atoms of each element present in the molecule.