Fossil fuels are the largest contributor of the ___________ gas carbon dioxide. this causes health and environmental issues.
question 2 options:
inert
greenhouse
poisonous
blue
Answers
Fossil fuels are the largest contributor of the greenhouse gas carbon dioxide,this causes health and environmental issues.
This causes health and environmental issues as it contributes to global warming and climate change. The burning of fossil fuels such as coal, oil and gas releases carbon dioxide into the atmosphere, which traps heat and leads to the Earth's temperature rising.
This can cause extreme weather events, rising sea levels, and harm to ecosystems and wildlife. Additionally, carbon dioxide can contribute to respiratory and cardiovascular health issues in humans and animals.
Therefore, it is important to transition to renewable energy sources in order to reduce our reliance on fossil fuels and mitigate the impacts of climate change.
To know more about global warming click on below link:
https://brainly.com/question/12908180#
#SPJ11
Related Questions
A sample of gas has a volume of 2.00L at 25.0 degrees Celcius and 1.08atm. What volume ( in liters) will it have at 100 degrees Celcius and 1.5atm?
Answers
Answer:
Explanation:
The question requires us to calculate the volume (in L) of a gas under the conditions given.
The following information was provided by the question:
Initial volume of gas = V1 = 2.00 L
Initial temperature of gas = T1 = 25.0 °C
Initial pressure of gas = P1 = 1.08 atm
Final temperature of gas = T2 = 100 °C
Final pressure of gas = P2 = 1.50 atm
To solve this problem, we'll need to apply the equation of ideal gases (shown below) twice: first, to determine the number of moles of gas, and again to calculate the volume of gas under the final conditions. Note that we'll determine the number of moles of gas under the initial conditions because this amount won't change (as we're talking about the same sample of gas).
\(undefined\)How many moles are in 79.6 grams of Fe2O3?
Answers
Answer:
0.49847139613321184
Explanation:
Fe2O3 in 1 mol is 159.6882.
Divide 76.9 by 159.6882.
Consider two kinesin motor proteins at the mitotic spindle midzone: kinesin-5 is a tetrameric motor that walks toward the plus end of both microtubules to which it is attached via its motor domains; kinesin-14, on the other hand, walks toward the minus end of one microtubule while it is attached to another microtubule via its tail domain. How do these motors affect the length of the spindle?.
Answers
At the microtubule midzone, two kinase motor proteins are present. The spindle is lengthened by kinesin-5 (kinesin A), whereas it is shortened by kinesin-14 (kinesin B).
How do proteins work?The body uses the large, complex molecules called proteins for a number of essential processes. They are crucial for the structure, operation, and control of the body's organs and tissues and carry out the majority of their job inside cells. an aesa of amino acids. Proteins are essential to the body's healthy operation.
What use does a protein serve?Protein is present in every human cell. Proteins' fundamental building block is an amino acid chain. Your body needs protein to replace destroyed cells and repair existing ones.
To know more about proteins visit:
https://brainly.com/question/24203058
#SPJ4
for the reaction below: a. estimate the gas phase enthalpy change using bond dissociation enthalpies from the owl table reference, not data from your text. click the references button and then click the tables link on the drop-down that appears. include algebraic sign and units. fill in the blank 1 b. is the reaction exothermic or endothermic? c. is the reaction likely to proceed spontaneously in the direction written? submit answer retry entire group 7 more group attempts remaining
Answers
a. The gas phase enthalpy change using bond dissociation enthalpies is - 641 kJ/mol.
b. The reaction is exothermic reaction.
c. Due negative enthalpy is the reaction likely to proceed spontaneously in the direction written.
The chemical equation is as :
Na + Cl--------> NaCl
The enthalpy change for the breaking of the bonds in the reaction is :
= (1 mol Na x 109 kJ/mol Na) + (1 mol Cl x 121 kJ/mol Cl)
= 230 kJ/mol
The enthalpy change for the formation of the bonds in NaCl is:
= 1 mol Na-Cl x (-411 kJ/mol Na-Cl)
= -411 kJ/mol
The overall enthalpy change is as :
ΔH = (enthalpy of products) - (enthalpy of reactants)
ΔH = (-411 kJ/mol) - (230 kJ/mol)
ΔH = -641 kJ/mol
The change enthalpy is -641 kJ/mol.
The reaction is the exothermic reaction because of the enthalpy change is the negative.
The chemical reaction is spontaneously in the direction in the written because of the negative enthalpy change.
To learn more about exothermic reaction here
https://brainly.com/question/28546817
#SPJ4
This question is incomplete, the complete question is :
for the reaction below: a. estimate the gas phase enthalpy change using bond dissociation enthalpies from the owl table reference, not data from your text. click the references button and then click the tables link on the drop-down that appears. include algebraic sign and units. fill in the blank 1 b. is the reaction exothermic or endothermic? c. is the reaction likely to proceed spontaneously in the direction written?
Na + Cl--------> NaCl
The standard unit for measuring volume is the _____.
milliliter
liter
deciliter
cubic centimeter
Answers
Which of these elements is COMMONLY found
in lipids?
A. hydrogen
B. iron
C. argon
D. helium
Answers
Answer:
A, Hydrogen
Explanation:
Let me know if I got this wrong! Hope this helps!
Lipids are organic molecules that consist mainly of carbon and hydrogen atoms. While some lipids may contain other elements, such as oxygen or nitrogen, hydrogen is the most abundant element found in lipids. This is because hydrogen atoms are essential components of the hydrocarbon chains that make up the backbone of many lipid molecules, such as fatty acids and triglycerides.
please help me ill give you brainliest
A lab procedure calls for 0.400 M NaOH solution. What volume would you end up with if you diluted 0.100 L of 0.700 M NaOH solution to obtain the necessary NaOH solution?
a. 0.0280 L
b. 0.0500 L
c. 5.21 L
d. 0.175 L
please help me ill give you brainliest

Answers
Describe how temperature changes
from the interior of the Sun through its
atmospheric layers.
Answers
Answer:
The Sun's temperature, which reaches around 15 million degrees Celsius in its core, steadily decreases with distance from the core, falling to 6000°C at its 'surface'. ... Instead, it rises to about 10,000°C in the chromosphere, and exceeds a million degrees Celsius in the corona.
Explanation:
Elements with unpaired electrons are:
Answers
Elements with unpaired electrons are known as paramagnetic elements. Paramagnetic elements, which have at least one unpaired electron in their outermost shell and can be easily influenced by an external magnetic field.
Paramagnetic elements are those which have at least one unpaired electron in their outermost shell. These unpaired electrons can be easily influenced by an external magnetic field and can become magnetized, thus exhibiting paramagnetism.
Hence, In summary, elements with unpaired electrons are referred to as paramagnetic elements, which have at least one unpaired electron in their outermost shell and can be easily influenced by an external magnetic field.
learn more about electrons click here:
https://brainly.com/question/860094
#SPJ11
What type of reactive intermediate is formed in the reaction of propene with N-bromosuccinimide to give 3-bromo-1-propene
Answers
Propene on reaction with N-bromosuccinimide in CCl4 produces 3-bromopropene.
In this reaction, allylic H atom is replaced with Br atom.
What are allylic radicals ?An allylic radical is the kind of reactive intermediate that is created when propene reacts with n-bromosuccinimide (nbs) to produce 3-bromo-1-propene. With NBS as one of the reactants, it is likely that a free radical bromination will take place.
The methyl group in propene would be attacked because the free radical that is forming can only be stabilized via resonance. A radical is said to be allylic if its resonance forms, which individually include unpaired electrons, are all located on an allylic carbon. Depending on where the allylic carbon is located, it can be categorized as a primary, secondary, or tertiary allylic radical.
To view more about the reactions, refer to:
https://brainly.com/question/4340058
#SPJ4
given the balanced equation for producing bromomethane: br2 ch4 - ch3br hbr identify the type of organic reaction shown.
Answers
Substitution reactions are one of the fundamental types of chemical reactions that involve the replacement of one atom or group of atoms by another atom or group of atoms. A substitution reaction can occur in organic or inorganic compounds.
The balanced equation for producing bromomethane is as follows:
Br2 + CH4 → CH3Br + HBr.
The type of organic reaction shown is a substitution reaction. In the above reaction, the hydrogen (H) atom in methane (CH4) is replaced by a bromine (Br) atom. The resulting product, bromomethane (CH3Br), is an organic halide that is commonly used as a methylating agent in organic synthesis.
Learn more about Substitution reactions here ;
https://brainly.com/question/30339615
#SPJ11
What is the balanced chemical reaction when Aluminium reacts with NaOH to produce NaAlO2 and H2 gas?
Answers
Answer:
2NaOH + 2Al +2H2O = 2NaAlO2 +3H
Explanation:
Aluminum is an amphoteric element it reacts with both bases and acids to form a salt and hydrogen gas.The reaction is highly exothermic.
2Al+6HBr=2 AlBr3+3H2
When 73.22 grams of Al reacts with 54.96 grams of HBr, what is the limiting reactant?
Answers
Taking into account the reaction stoichiometry and the definition of limiting reactant, HBr is the limiting reagent.
Reaction stoichiometryIn first place, the balanced reaction is:
2 Al + 6 HBr → 2 AlBr₃ + 3 H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al: 2 molesHBr: 6 molesAlBr₃: 2 molesH₂: 3 molesThe molar mass of the compounds is:
Al: 27 g/moleHBr: 80.9 g/moleAlBr₃: 266.7 g/moleH₂: 2 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al: 2 moles ×27 g/mole= 54 gramsHBr: 6 moles ×80.9 g/mole= 485.4 gramsAlBr₃: 2 moles ×266.7 g/mole= 533.4 gramsH₂: 3 moles ×2 g/mole= 6 gramsDefinition of limiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 54 grams of Al reacts with 485.4 grams of HBr, 73.22 grams of Al reacts with how much mass of HBr?
mass of HBr= (73.22 grams of Al×485.4 grams of HBr)÷54 grams of Al
mass of HBr= 658.166 grams
But 658.166 grams of HBr are not available, 54.96 grams are available. Since you have less mass than you need to react with 73.22 grams of Al, HBr will be the limiting reagent.
Learn more about limiting reactant:
https://brainly.com/question/19033878
#SPJ1
C5H12(g) + O2(g) → CO2(g) + H₂O(g)
Classify the reaction
Answers
The combustion reaction is the process by which a chemical substance or hydrocarbon reacts with oxygen to produce carbon dioxide and water while also releasing energy in the form of light and heat.
What is combustion reaction?When a chemical substance interacts with oxygen to create carbon dioxide and water, a combustion process occurs and energy is released. O2 must be one of the reactants in it.An illustration of a combustion reaction is the burning of wood or coal indoors during the winter.Another illustration of a combustion reaction is the generation of energy in thermal power plants and the burning of gasoline and diesel in automobiles.An illustration of a general combustion reaction is as follows:O2 + CnH2n = nCO2 + nH2OFor more information on combustion reaction kindly visit to
https://brainly.com/question/12172040
#SPJ1
37. Between 02, SO, and H20, which would have the highest vapor pressure? *Helppppp

Answers
First, remember that:
The vapor pressure of a liquid is directly related to the intermolecular forces present between its molecules. If these forces are stronger, the evaporation rate and the vapor pressure will be lower.
So, we're going to analyze the intermolecular forces of each element:
O2: The molecule of oxygen consist of two oxygen atoms bonded together by a double bond. It consist of just London dispersion forces, so this is a really weak force.
H2O: Water has hydrogen bonds, dipole-induced dipole forces, and London dispersion forces. So it has strong forces.
SO: It consists of London dispersion forces, and there's a difference of electronegativity between S and O, so there's a greater force than the force of O2.
Therefore, O2 could have the highest vapor pressure, as its intermolecular forces are the weakest.
explain the process of vulcanization(help im in class8)!!!
Answers
Explanation:
a chemical process in which the rubber is heated with sulphur, accelerator and activator at 140–160°C. The process involves the formation of cross-links between long rubber molecules so as to achieve improved elasticity, resilience, tensile strength, viscosity, hardness and weather resistance.
Answer:
Vulcanization is a chemical process in which the rubber is heated with sulphur, accelerator and activator at 140–160°C. The process involves the formation of cross-links between long rubber molecules so as to achieve improved elasticity, resilience, tensile strength, viscosity, hardness and weather resistance.
Explanation:
Question 4
10 pts
470 mL of gas is at 56.9 C. What does the temperature (in Kelvin) need to
be in order for the volume to be 61.3 mL?
Round to 2 decimal places
Answers
Answer:
5.0
Explanation:
Electronegativity is a measure of:
a. an atom's ability to pull protons to itself.
b. an atom's ability to form ionic bonds.
c. an atom's ability to form covalent bonds.
d. an atom's ability to pull bonded electrons to itself.
Answers
Electronegativity is a measure of an atom's ability to pull bonded electrons to itself.
The correct answer is d.
It is a relative scale that quantifies the attraction an atom has for electrons in a chemical bond. A highly electronegative atom has a stronger pull on the shared electrons, resulting in an uneven distribution of electron density within the bond.
This can lead to the formation of polar covalent bonds, where there is a partial positive charge on one atom and a partial negative charge on the other. Electronegativity is an important concept in understanding chemical bonding, as it helps predict the nature of bonds, such as whether they are nonpolar covalent, polar covalent, or ionic.
Learn more about Electronegativity:
brainly.com/question/18258838
#SPJ11
SCIENCE ASSAP
Genetic Diversity
• The variation in genes that exists within a species
• Organisms with a large gene pool have a greater
chance of surviving and flourishing than a
population with limited genetic variability.
• Ex: resistance to disease, tolerance to cold
• Darwin termed this process "natural selection."
To the left you can see the variety of genes that exist
in types of fruit. Can you think of another species
that has similar variations?
Kesler Science, LLC
Answers
Answer:
Oranges
Explanation:
Oranges have a lot of genetic diversity within them, since oranges were already well versed in growing in winter months, oranges have gentically modified themselves through natural selection into be able to prouduce in warmer climates. Oranges also have changes in genetics which gives them a lot of gentic diversity that effects their, size, taste, texture, acidity and ability to reproduce.
the half-life for the transmutation of radon-222 () to lead-214 () is 3.8 days. if there is an initial mass of 100.0 g of radon-222, how much radon-222 would remain after 1.9 days?
Answers
The half-life for the transmutation of radon-222 that would remain after 1.9 days is approximately 70.71 g.
To calculate the transmutation of radon-222 that would remain after 1.9 days, we can use the concept of half-life.
Half-life of radon-222 (t½) = 3.8 days
Initial mass of radon-222 = 100.0 g
Time elapsed (t) = 1.9 days
The number of half-lives elapsed can be calculated as:
Number of half-lives (n) = t / t½
Substituting the values:
n = 1.9 days / 3.8 days = 0.5
Now, we can calculate the remaining mass of radon-222 using the formula:
Remaining mass = Initial mass × (0.5)^n
Substituting the values:
Remaining mass = 100.0 g × (0.5)^0.5 ≈ 70.71 g
Therefore, approximately 70.71 g of radon-222 would remain after 1.9 days.
Learn more about half-life from the given link:
https://brainly.com/question/25750315
#SPJ11
The carboxylic acid contains six carbons and the starting material should be an alkyl halide of five carbons or less, so you should look for a method of generating carboxylic acids by adding a carbon. One method of synthesizing carboxylic acids is from the hydrolysis of nitriles, which can be formed by substitution of alkyl halides. Identify the structure that contains a nitrile. RCH=CHNH, OCH,CEN OCH,CH=NH OCH,CH, NH
Answers
The synthesis of carboxylic acid can be done by hydrolysing the nitrile which is substituted in place of halide by using cyanide ion. The structure that contains a nitrile is OCH2C≡N.
A nitrile is an organic compound that contains a carbon triple bonded to a nitrogen atom.
The general formula for a nitrile is R–C≡N, where R is an alkyl or aryl group. In the structure OCH2C≡N, the OCH2 group is the alkyl group and the C≡N is the nitrile functional group. Therefore, this structure contains a nitrile.
To synthesize a carboxylic acid from an alkyl halide, you can first convert the alkyl halide to a nitrile through a substitution reaction with a cyanide ion. The nitrile can then be hydrolyzed to form a carboxylic acid. The overall reaction is:
R–X + CN– → R–C≡N → R–COOH
Where R is an alkyl or aryl group, X is a halogen, and CN– is a cyanide ion.
In conclusion, the structure that contains a nitrile is OCH2C≡N and it can be used to synthesize a carboxylic acid from an alkyl halide through a substitution reaction followed by hydrolysis.
To know more about carboxylic acid, refer here:
https://brainly.com/question/29035899#
#SPJ11
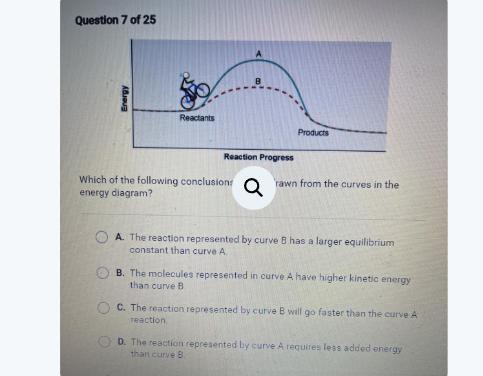
Energy
Reactants
Products
Reaction Progress
Which of the following conclusions can be drawn from the curves in the
energy diagram?
OA. The reaction represented by curve B has a larger equilibrium
constant than curve A.
▸
B. The reaction represented by curve A requires less added energy
than curve B.
OC. The molecules represented in curve A have higher kinetic energy
than curve B.
OD. The reaction represented by curve B will go faster than the curve A
reaction.

Answers
The correct answer is that The reaction represented by curve B will go faster than the curve A reaction.
The energy diagramAn energy diagram illustrates the transfer or utilization of energy. In particular, it depicts the change in energy during a chemical reaction and is sometimes referred to as a reaction progress curve.
In the given energy diagram, there is a representation of a person bicycling. Notably, curve A is positioned higher than curve B. This height difference indicates that bicycling along curve A requires a greater amount of energy compared to bicycling along curve B. Conversely, curve B necessitates a lower amount of energy for the bicycling activity.
Read mroe on energy diagram here https://brainly.com/question/21047184
#SPJ1
what is the heat of solution (qsoln) for the neutralization reaction between nh3 and hcl?
Answers
The heat of solution (qsoln) for the neutralization reaction between nh3 and hcl can be calculated using the heat of neutralization equation: qsoln = -qrxn/moles of solute. The reaction between nh3 and hcl is an exothermic reaction, meaning it releases heat. The balanced chemical equation for the reaction is:
NH3 (aq) + HCl (aq) → NH4Cl (aq)
To calculate qsoln, we need to know the amount of heat released (qrxn) and the number of moles of solute. The heat of neutralization for this reaction is -51.4 kJ/mol. This means that for every mole of NH3 and HCl that react, 51.4 kJ of heat are released. To determine the number of moles of solute, we need to know the amount of NH3 and HCl present in the reaction.
Let's say we have 100 mL of a 0.1 M NH3 solution and we add 50 mL of a 0.1 M HCl solution to it. The total volume of the solution is 150 mL, and the total number of moles of NH3 and HCl is:
moles of NH3 = (0.1 mol/L) x (0.1 L) = 0.01 moles
moles of HCl = (0.1 mol/L) x (0.05 L) = 0.005 moles
The limiting reactant is HCl, so we can use its moles to calculate qsoln:
qsoln = -qrxn/moles of solute
qsoln = -(-51.4 kJ/mol)/(0.005 mol)
qsoln = 10,280 kJ/mol
Therefore, the heat of solution (qsoln) for the neutralization reaction between NH3 and HCl is 10,280 kJ/mol.
Learn more about neutralization reaction here:
brainly.com/question/28970253
#SPJ11
How many Coulombs are in 4×10
4
electrons? (6×10
−15C
)
Answers
There are 6.4 × 10⁻¹⁵ Coulombs in 4 × 10⁴ electrons.
To convert the number of electrons to coulombs, we need to first multiply the number of electrons by the charge of a single electron
No. of electrons × Charge of single electron
Charge of single electron = 1.6 × 10⁻¹⁹ coulombs
Calculating using the above formula
we get: 4 × 10^4 electrons × 1.6 × 10⁻¹⁹ C/electron = 6.4 × 10⁻¹⁵ Coulombs
To know more about Electrons refer to this link
https://brainly.in/question/374445
Which statement correctly describes
spontaneous generation?
O the idea that life can arise from something nonliving
O the duplication of cells from other cells
O the generation of molecules from exposure to sunlight
O the production of energy from an unknown source
XEdgenuity
Answers
Answer:
The idea that life can arise from something nonliving
Explanation:
I got the answer right
Answer: The first answer
Explanation:
I've done this question before.
hydrogen peroxide h2o2 decomposes to form water and oxygen this reaction is thermodynamically favorite at room temperature
Answers
Decomposition of hydrogen peroxide has a thermodynamic instability. It decomposes at higher temperatures and concentrations to form water and oxygen. Other compounds, such as transition metals like silver and platinum, can catalyze the decomposition of hydrogen peroxide.
In the absence of a catalyst, the reaction of hydrogen peroxide decomposition is very slow at moderate temperatures. It can be accelerated by raising the temperature, which initiates the thermal decomposition reaction. This reaction can take place in either the liquid or vapour phase.
\(H_{2} O_{2}\) is the chemical formula for hydrogen peroxide. It is a very pale blue liquid that is slightly more viscous than water in its pure form. It is used as an oxidizer, bleaching agent, and antiseptic in water, usually in a dilute solution (3%-6% by weight) for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, also known as "high-test peroxide," decomposes explosively when heated and has been used as a rocket propellant.
To learn more about decomposition of hydrogen peroxide, here
https://brainly.com/question/19129412
#SPJ4
The isotope 226Ra has a half-life for radioactive decay of 1600 Y. How long will it take the amount of 226 Ra in a sample of 226RaCl2 to decrease by 25%
Answers
The Liquified Petroleum Gas (LPG) has the composition of 60% Propane (C 3
H 8
) and 40% Butane (C 4
H 10
) by volume: (a) Find the wet volumetric and gravimetric analysis of the products of combustion when the equivalence ratio (Φ)=1.0. (b) What is the stoichiometric air to fuel ratio for the LPG.
Answers
The balanced combustion reaction for propane can be represented as:
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
And the balanced combustion reaction for butane can be represented as:
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
Since LPG is composed of 60% propane and 40% butane by volume, we can calculate the wet volumetric and gravimetric analysis based on these proportions.
Wet volumetric analysis:
For the wet volumetric analysis, we consider the volume of the products of combustion relative to the volume of the LPG consumed.
Propane (C₃H₈):
The stoichiometric coefficient of propane in the combustion reaction is 3. Therefore, for every mole of propane burned, we will have 3 moles of CO₂ and 4 moles of H₂O formed.
Butane (C₄H₁₀):
The stoichiometric coefficient of butane in the combustion reaction is 4. Therefore, for every mole of butane burned, we will have 4 moles of CO₂ and 5 moles of H₂O formed.
Considering the initial composition of 60% propane and 40% butane by volume, we can calculate the volumetric composition of the products of combustion:
Volumetric composition of CO₂:
(0.6 * 3) + (0.4 * 4) = 3.6
Volumetric composition of H₂O:
(0.6 * 4) + (0.4 * 5) = 4.6
Therefore, the wet volumetric analysis of the products of combustion is 3.6 parts CO₂ to 4.6 parts H₂O.
Wet gravimetric analysis:
For the wet gravimetric analysis, we consider the mass of the products of combustion relative to the mass of the LPG consumed.
Using the molar masses of the compounds involved in the combustion reaction:
Molar mass of CO₂ = 44 g/mol
Molar mass of H₂O = 18 g/mol
Gravimetric composition of CO₂:
(0.6 * 3 * 44 g/mol) + (0.4 * 4 * 44 g/mol) = 158.4 g
Gravimetric composition of H₂O:
(0.6 * 4 * 18 g/mol) + (0.4 * 5 * 18 g/mol) = 74.4 g
Therefore, the wet gravimetric analysis of the products of combustion is 158.4 grams CO₂ to 74.4 grams H₂O.
(b) The stoichiometric air to fuel ratio for LPG can be determined based on the balanced combustion equations for propane and butane.
For propane (C₃H₈):
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
The stoichiometric coefficient for propane is 1, which means we need 5/2 moles of O₂ for every mole of propane.
For butane (C₄H₁₀):
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
What is the windward side of the sand dune

Answers
Answer:
A dunes windward side is the side where the wind is blowing and pushing material up. A dunes slip face is simply the side without wind. A slipface is usually smoother than a dunes windward side. A collection of dunes is called a dune belt or dune field.
Explanation:
Hope this helps...
hydrogen, h2 draw the molecule by placing atoms on the grid and connecting them with bonds. include nonbonding electrons.
Answers
Hydrogen typically has 0 non-bonding electrons. The full valence shell for hydrogen is 2 and the number of electrons in bonds is also 2. The difference is zero.
What is non-bonding electron?A non-bonding electron is an electron not involved in chemical bonding. It takes two electrons to form a covalent bond, we can calculate the number of non-bonding electrons in the molecule by subtracting two electrons from the total number of valence electrons for each bond in the skeleton structure. In hydrogen molecule, both hydrogen atoms share the two electrons in the covalent bond, and each acquires a helium-like electron configuration.
learn more about non-bonding electrons: https://brainly.com/question/18258856
#SPJ4
