How many moles of chlorine gas react when 320. 5 grams of aluminum chloride is formed?
Answers
To determine the number of moles of chlorine gas required for the formation of 320.5 grams of aluminum chloride, we need to use the balanced chemical equation for the reaction. The equation for the reaction between aluminum and chlorine gas to form aluminum chloride is:
2Al(s) + 3Cl2(g) → 2AlCl3(s)
From the equation, we can see that for every two moles of aluminum, three moles of chlorine gas are required to form two moles of aluminum chloride. Therefore, we can set up a proportion:
2 moles of AlCl3 : 3 moles of Cl2 = 320.5 g of AlCl3 : x
Where x is the number of moles of Cl2 required.
We can use the molar mass of aluminum chloride (133.34 g/mol) to convert the mass of AlCl3 to moles:
320.5 g AlCl3 ÷ 133.34 g/mol = 2.403 moles AlCl3
Substituting the values into the proportion, we get:
2 moles of AlCl3 : 3 moles of Cl2 = 2.403 moles of AlCl3 : x
Solving for x, we get:
x = 3.605 moles of Cl2
Therefore, 3.605 moles of chlorine gas are required to react with 320.5 grams of aluminum to form aluminum chloride.
To know more about moles of chlorine gas required refer here
https://brainly.com/question/29188808#
#SPJ11
Related Questions
a high school student was splashed in the eyes with a strong acid chemical during a lab experiment. he is in severe pain and is unable to open his eyes. you should:
Answers
high school student was splashed in the eyes with a strong acid chemical during a lab experiment you should Immediately call for emergency medical services by dialing 911 or the local emergency number.
Some more precaution should be taken lab experiment
While waiting for medical help to arrive, assist the student in removing any contaminated clothing and flush the affected area with water for at least 20 minutes, using a continuous, gentle stream of water.
Continue flushing the eyes with water until medical help arrives, and keep the student calm and reassured.
Do not attempt to neutralize the acid with any other substances or solutions, as this can cause further damage to the eyes and skin.
If available, consult the safety data sheet (SDS) for the chemical to obtain specific first aid instructions.
Follow any additional instructions or protocols provided by the school or the laboratory, including reporting the incident to school officials and documenting the incident.
To know more about lab visit:
brainly.com/question/31529120
When a high school student is splashed in the eyes with a strong acid during a lab experiment you should call for help, wash the eye with water, seek medical attention and stay calm.
1. Stay calm: Remaining composed will help you provide appropriate assistance and ensure the student's safety.
2. Call for help: Notify a teacher or lab supervisor immediately, as they can provide further guidance and support.
3. Locate an eyewash station: If available, guide the student to an eyewash station, which is designed for emergencies like this. If an eyewash station is not available, use a water source with a gentle stream.
4. Flush the eyes: Have the student lean over the water source, and gently but continuously flush their eyes with clean water. Encourage them to open their eyes as much as possible to facilitate thorough rinsing. Continue this process for at least 15-20 minutes to neutralize and remove the acid.
5. Seek medical attention: Once the initial flushing is complete, seek immediate medical attention for the student, even if their symptoms seem to improve. Acid exposure can cause lasting damage, and it's important for a medical professional to evaluate the extent of the injury and provide appropriate care.
6. Document the incident: After the student has received medical care, document the details of the incident, including the chemical involved and any safety measures in place. This information can be helpful for both medical professionals and school administrators in understanding and addressing the situation.
Know more about medical attention here:
https://brainly.com/question/31087759
#SPJ11
Question 1
1 pts
How many grams of sodium is contained in the final container when you dispense 564.2 mL of a
5.72 M sodium chloride solution into a beaker?
The atomic mass of sodium is 22.99 amu
The atomic mass of chlorine is 35.45 amu
Write your answer without units.
Next
Answers
Therefore, there are 73.3 grams of sodium in the final container.
Is sodium chloride acidic or basic?Sodium chloride (NaCl) is a neutral compound, meaning it is neither acidic nor basic. It is a salt formed by the combination of sodium (Na+) and chloride (Cl-) ions, which have a neutral charge and therefore do not affect the pH of a solution.
Firstly, the number of moles of sodium in the solution will be:
n = C * V = 5.72 M * 564.2 mL = 3.21 moles
Next, we convert the number of moles of sodium to grams:
mass = n * atomic mass = 3.21 moles * 22.99 amu/mole = 73.3 grams
To know more about sodium visit :-
brainly.com/question/29327783
#SPJ1
Two graduated cylinders, one filled with water to 20 millimeters and one containing a rock and water filled to 32 millimeters.
Use the image to determine the volume of the rock.
Initial volume:
mL
Final volume:
mL
Volume of rock:
cm3
Answers
Answer:
1. 20
2. 32
3. 12
Explanation:
PLEASE HELP ASAP
Show all work and include units when necessary for full credit!
An isotope of element X has a mass of 12.32 amu and a relative abundance of 19.5%; a second isotope has a mass of 13.08 amu and a relative abundance of 26.23%; the third isotope in the sample has a mass of 11.99 amu and a relative abundance of 54.27%. Calculate the atomic mass of element X
Answers
Answer:
12.34 amu
Explanation:
Let the 1st isotope be A
Let the 2nd isotope be B
Let the 3rd isotope be C
From the question given above, the following data were obtained:
1st Isotope (A):
Mass of A = 12.32 amu
Abundance (A%) = 19.5%
2nd isotope (B):
Mass of B = 13.08 amu
Abundance (B%) = 26.23%
3rd isotope (C):
Mass of C = 11.99 amu
Abundance (C%) = 54.27%
Atomic mass of X =?
The atomic mass of the element X can be obtained as follow:
Atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100] + [(Mass of C × C%)/100]
= [(12.32 × 19.5)/100] + [(13.08 × 26.23)/100] + [(11.99 × 54.27)/100]
= 2.402 + 3.431 + 6.507
= 12.34 amu
Thus, the atomic mass of the element X is 12.34 amu
Which two substances are reactants in the following chemical equation?
CO + H₂O CO₂ + H₂
A. H₂0
■ в. со
C. CO₂
OD. H₂
Answers
Because these compunds of reactant and the compounds of the product . The reactant goint to equation
After reduction with LiAlH4, followed by aqueous workup (protonation) the following starting materials will form what?
Carboxylic acids
Esters
Amides
Answers
After reduction with LiAlH4, followed by aqueous workup (protonation), carboxylic acids, esters, and amides will form their corresponding alcohols, primary alcohols for carboxylic acids and esters, and secondary alcohols for amides.
LiAlH4 is a strong reducing agent that is capable of reducing carbonyl functional groups, which include carboxylic acids, esters, and amides, to their corresponding alcohols. The aqueous workup (protonation) serves to neutralize the reaction mixture and remove any remaining LiAlH4. The resulting alcohols can then be further reacted or purified as desired.
After reduction with LiAlH4 (lithium aluminum hydride), followed by aqueous workup (protonation), the starting materials will undergo the following transformations:
1. Carboxylic acids: Reduction with LiAlH4 will convert carboxylic acids into primary alcohols.
2. Esters: LiAlH4 reduction will convert esters into a pair of alcohols, including a primary alcohol from the carbonyl group and an alcohol derived from the ester's alkyl/aryl group.
3. Amides: Reduction using LiAlH4 will transform amides into amines.
In summary, LiAlH4 is a strong reducing agent that can reduce carboxylic acids, esters, and amides to their corresponding alcohols or amines through the process of reduction followed by aqueous workup.
For more information on LiAlH4 visit:
brainly.com/question/30904954
#SPJ11
A 125 cm3 soap bubble is formed outside, where the temperature is 10.0°C. It drifts through an open door, expands and pops in a house. If the maximum volume of the bubble could be 140 cm3, what is the temperature inside the house? (In C°)
Answers
In this question, we have a situation where a gas is in constant pressure but changing its volume and temperature, and the best way to solve a situation like this, is through the Charles's gas law formula, which shows the relationship between volume and temperature when the pressure is constant. The formula is:
V1/T1 = V2/T2
We have:
V1 = 125 cm3, or 0.125 Liters
T1 = 10.0°C, or 283 K
V2 = 140 cm3, or 0.140 Liters
T2 = ?
Now we add these values into the formula:
0.125/283 = 0.140/T2
0.000442 = 0.140/T2
T2 = 0.140/0.000442
T2 = 317 K, or 44°C
when holden goes to visit phoebe, how does she react to his arrival? how are the two similar and how are they different?
Answers
Catcher in the Rye is a young-adult fiction by J. Salinger. It is a novel based on the coming of the age and literary realism. Phoebe is disappointed with Holden's vindication.
Holden Caulfield is the protagonist and the narrator of the story who is intelligent but is expelled from the school. Phoebe is Holden's sister, she is infuriated by Holden's dismissal.
She knows that their father would be angry because Holden is expelled from school. Holden tries to explain his situation to his sister and tells her about his minimum chance of joining the military school.
He explains to her how bad is his school Pencey and he dislikes that place. But Phoebe interrupts him by saying that he doesn't like anything or anywhere.
Her challenge makes him think about the one thing that he likes the most and he couldn't. It shows Phoebe's concern to stimulate Holden. She is seen sympathetic and angry with her brother for not growing up.
Holden describes them in loving, caring terms, demonstrating his genuine affection and respect for them. How does Holden characterize Phoebe? Holden describes Phoebe as wise, overly affectionate at times, and overly emotional for her age.
Also as smart, tiny, and a little noisy. Holden, Phoebe, and Allie are siblings in the novel "The Catcher in the Rye." Holden looks up to his younger brother and sister as idealized versions of himself. Holden describes Allie as the most intelligent and pleasant member of his family.
To know more about the Holden, refer: https://brainly.com/question/1344932
#SPJ4
is water?
A. a substance made up of two different elements (hydrogen and oxygen)
B. an element

Answers
Answer:
A substance made up of two different elements (hydrogen and oxygen)
Can someone help me with this please ASAP?

Answers
The reaction between C₂H2O, and O₂ is represented by the balanced equation above. In an experiment, 0.30 mol of CO₂ was produced from the reaction of 0.05 mol of C₂H₂O with excess
O₂. The reaction was repeated at the same temperature and in the same container, but this time 0.60 mol of CO₂ was produced. Which of the following must be true?
Answers
There must have been 0.10mol of \(C_{2} H_{2} O\) in the container at the beginning.
\(C_{2} H_{2} O\) + \(2O_{2}\) = \(2CO_{2}\) + \(H_{2} O\)
The above reaction makes it quite evident that 1 mol of \(C_{2} H_{2} O\) combines to create 2 mol of \(CO_{2}\) and \(O_{2}\) is given in excess that \(C_{2} H_{2} O\) alone controls a product's formation. Therefore, here, O is an excess reactant and \(C_{2} H_{2} O\) is a limiting reactant.
It takes 6 times as much \(C_{2} H_{2} O\) to produce 1 mol of \(CO_{2}\) from 0.05 mol of \(C_{2} H_{2} O\).
Now, 0.6 divided by 6 mol of reactant is required for 0.60 moles of \(CO_{2}\)to produce, which translates to
moles of \(C_{2} H_{2} O\) = 0.6/6 = 0.1 mol .
Therefore, \(C_{2} H_{2} O\) must have been present in the container in an initial concentration of 0.10mol.
Learn more about mole calculations here-
https://brainly.com/question/1578931
#SPJ9
Draw the products formed when pentanoic anhydride [(CH_3CH_2CH_2CH_2CO)_2O] is treated with the following reagent. Differentiate products by greater or lesser molecular mass.
Answers
The (\(CH_3CH_2CH_2CH_2COONa\)) and sodium carbonate (\(Na_2CO_3\)). The sodium pentanoate has a lower molecular mass than the sodium carbonate.
What is sodium carbonate?Sodium carbonate is a chemical compound with the formula Na2CO3. It is a sodium salt of carbonic acid and has a strong alkaline taste. It can be found naturally or produced synthetically, and is commonly used in a wide range of industrial and household applications. As a food additive, it is used to regulate acidity and as a preservative. Additionally, it is used in the production of glass, textiles, soaps and detergents, in water treatment, and as an ingredient in various cleaning products. Its natural form, known as natron, is found in mineral deposits and has been used since ancient times. Sodium carbonate is an important industrial chemical, used in a variety of industries for a variety of purposes.
To learn more about sodium carbonate
https://brainly.com/question/31016310
#SPJ4
![Draw the products formed when pentanoic anhydride [(CH_3CH_2CH_2CH_2CO)_2O] is treated with the following](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/7Fx2lD7gMGneZ04m0khw1p78DMWiDJNe.png)
A metalloid acts as a conductor at what temperature
Answers
Answer: high temperatures
Explanation:
It varies.
What has a complete ionic equation of Mg(s) + Zn2+ + 2NO3 → Mg2+ + 2NO3 + Zn(s)?
O A. Mg(s) + Zn2+ → Mg2+ + Zn(s)
O B. Mg(s) + Zn(NO3)2(aq) + Mg(NO3)2(aq) + Zn(s)
O C. Mg(NO3)2 + Zn(s) → Zn2+ + 2NO3 + Mg(s) O D. Mg(s) + 2Zn(NO3)(aq) + 2MgNO3(aq) + Zn(s)
Answers
Answer: \(Mg(s)+Zn(NO_3)_2(aq)\rightarrow Mg(NO_3)_2(aq)+Zn(s)\)
Explanation:
Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and present in an aqueous are represented in the form of ions.
Net ionic equation : In the net ionic equations, we do not not include the spectator ions in the equations.
Spectator ions are the ions present on reactant and product side which do not participate in a reactions. The same ions present on both the sides.
The equation given is:
\(Mg(s)+Zn^{2+}+2NO_3^-\rightarrow Mg^{2+}+2NO_3^-+Zn(s)\)
The complete molecular equation will be:
\(Mg(s)+Zn(NO_3)_2(aq)\rightarrow Mg(NO_3)_2(aq)+Zn(s)\)
The complete ionic equation of\(\rm Mg(s) + Zn^{2+} + 2NO_3 \rightarrow Mg^{2+} + 2NO_3 + Zn(s)\) is\(\rm Mg(s) + Zn^{2+} \rightarrow Mg^{2+} + Zn(s)\). So, option A is right.
A complete ionic equation shows all of the ions that are present in a reaction, including the spectator ions. In this reaction, magnesium metal (Mg(s)) reacts with zinc(II) ions (\(\rm Zn^2+\)) to form magnesium(II) ions (\(\rm Mg2+\)) and zinc metal (Zn(s)).
The balanced molecular equation for this reaction is:
\(\rm Mg(s) + Zn(NO_3)_2(aq) \rightarrow Mg(NO_3)_2(aq) + Zn(s)\)
The complete ionic equation is:
\(\rm Mg(s) + Zn^{2+}(aq) \rightarrow Mg^{2+}(aq) + Zn(s)\)
The spectator ions are the nitrate ions (\(\rm NO_3^-\)), which are present on both sides of the equation. They do not participate in the reaction, so they are not included in the complete ionic equation.
The other choices are incorrect because they do not show all of the ions that are present in the reaction.
Therefore, the answer is A. \(\rm Mg(s) + Zn^{2+} \rightarrow Mg^{2+} + Zn(s)\).
Learn more about ions here;
https://brainly.com/question/30663970
#SPJ6
Conjugation requires ______ orbitals on three or more adjacent atoms in a structure.
Answers
Conjugation requires p orbitals on three or more adjacent atoms in a structure.
What is conjugation?The overlap of one p orbital with another over an adjacent bond is referred to as conjugation (in transition metals d orbitals can be involved). A conjugated system in a molecule is a system of connected p orbitals with delocalized electrons that reduces the overall energy of the molecule and promotes stability. It is typically represented by alternating single and multiple bonds. The system, which can be cyclic, acyclic, linear, or mixed, may contain lone pairs, radicals, or carbenium ions. Johannes Thiele, a German chemist, created the term "conjugated" in 1899. A conjugated system has a band of overlapping p orbitals that bridges the interjacent places where simple diagrams show no link.
To learn more about conjugation , visit:
https://brainly.com/question/15579188
#SPJ4
A chemical reaction can be concisely represented by a chemical _____. The substances that undergo a chemical change are the_____ The new substances formed are the _____.
Answers
A chemical reaction can be the concisely represented by a chemical equation. The substances that undergo the chemical change are the reactant . The new substances formed are the product .
The chemical reaction is represented by the chemical equation. The substance that will going to react or that undergo the chemical change are called as the reactant. The new substance that are formed by the chemical reaction are called as the product. The balance chemical equation is in which the toms in the reactant side is equals to the product side.
Thus, the substance undergo chemical change is the reactant and the new substance of formed are the products.
To learn more about chemical reaction here
https://brainly.com/question/10299842
#SPJ4
The electrons of an atom occupy one or more areas of space called ions. regions. nuclei. bonds. shells.
Answers
The electrons of an atom occupy one or more areas of space called shells. The shells, also known as energy levels, are regions around the nucleus where electrons are most likely to be found. Each shell has a maximum number of electrons it can hold, with the innermost shell being able to hold a maximum of 2 electrons, and subsequent shells being able to hold more.
Ions, on the other hand, are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. When an atom loses electrons, it becomes a positively charged ion called a cation. Conversely, when an atom gains electrons, it becomes a negatively charged ion called an anion.
Regions, nuclei, and bonds are not terms directly related to the areas of space occupied by electrons. Regions refers to general spaces or areas, whereas nuclei refers to the central part of an atom that contains protons and neutrons. Bonds, on the other hand, refer to the forces of attraction that hold atoms together in molecules.
Therefore, the correct term to describe the areas of space occupied by electrons is shells.
To know more about shells visit-
https://brainly.com/question/13514474
#SPJ11
H 0
:p A
=0.40,p B
=0.40, and p C
=0.20 H a
: The population proportions are not p A
=0.40,p B
=0.40, and p C
=0.20. sample of size 200 yielded 140 in category A, 20 in category B, and 40 in category C. Use α=0.01 and test to see whether the proportions are as stated in H 0
. (a) Use the p-value approach. Find the value of the test statistic. Find the p-value. (Round your answer to four decimal places.) p-value = State your conclusion. Reject H 0
. We conclude that the proportions differ from 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions are equal to 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions differ from 0.40, 0.40, and 0.20. Reject H 0
. We conclude that the proportions are equal to 0.40,0.40, and 0.20. (b) Repeat the test using the critical value approach. Find the value of the test statistic. State the critical values for the rejection rule. (If the test is one-tailed, enter NONE for the unused tail. Round your answers to three decimal places.) test statistic ≤ test statistic ≥ State your conclusion. Reject H 0
. We conclude that the proportions differ from 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions differ from 0.40, 0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions are equal to 0.40,0.40, and 0.20. Reject H 0
. We conclude that the proportions are equal to 0.40,0.40, and 0.20.
Answers
The test results indicate that we reject the null hypothesis (H0) and conclude that the proportions are not equal to 0.40, 0.40, and 0.20.
What is the value of the test statistic and the p-value for the given hypothesis test?To test the hypothesis, we can use the p-value approach. The test statistic for comparing proportions is the chi-square statistic (χ²). In this case, we have three categories (A, B, and C) and their respective observed frequencies (140, 20, and 40) in a sample of size 200.
The expected frequencies under the null hypothesis can be calculated by multiplying the sample size by the hypothesized proportions. Thus, the expected frequencies are 80 for category A, 80 for category B, and 40 for category C.
Using these observed and expected frequencies, we can calculate the chi-square test statistic:
χ² = Σ[(observed - expected)² / expected]
After calculating the test statistic, we can find the p-value associated with it using the chi-square distribution with degrees of freedom equal to the number of categories minus 1.
Comparing the p-value to the significance level (α = 0.01), if the p-value is less than α, we reject the null hypothesis; otherwise, we fail to reject it.
Learn more about null hypothesis
brainly.com/question/30821298
#SPJ11
Is the formation of CO2(g) from its elements endothermic or exothermic?
Exothermic because the ΔH is negative.
Endothermic because the ΔH is negative.
Endothermic because the ΔH is positive.
Exothermic because the ΔH is positive.
Answers
Answer: Exothermic because the ΔH is negative.
Explanation:
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements, with all substances in their standard states.
\(C(s)+O_2(g)\rightarrow CO_2(g)\)
Exothermic reactions are those reactions in which heat is released and \(\Delta H\) is negative.Endothermic reactions are those reactions in which heat is absorbed and \(\Delta H\) is positive.
The reaction is exothermic as the heat is released during the formation of \(CO_2\) as heat of formation of \(CO_2\) is negative.
reckless endangerment of human life what type of irony is used
Answers
The type of irony used in "reckless endangerment of human life" is verbal irony. Verbal irony is a figure of speech in which words are used to mean something different from their literal meaning.
In this instance, the phrase "reckless endangerment of human life" refers to behavior that puts people's lives in danger. However, it is ironic because it is a criminal offense that should be avoided and yet it is taking place. Verbal irony is often used for humorous or dramatic effect. This type of irony is used to create a contrast between what is said and what is meant. In this case, the phrase "reckless endangerment of human life" is used to describe behavior that is extremely dangerous, yet it is ironic because it is the opposite of what should be happening.
To learn more about Verbal irony check the link below-
https://brainly.com/question/1551288
#SPJ11
Calculate the force needed to accelerate a 125 kg object at 4.50 N/kg
Answers
Answer:
129.50 N/kg
Explanation:
125 kg + 4.50 N
129.50 N/kg
Which of the following is an easy and sustainable way for campuses to reduce waste?
a. recycling bins
b. canals for dumping waste
c. Incinerators for garbage
d. outsource to companies that specialize in recycling
Answers
Answer:
a recycling bins
Explanation:
it's the easiest to do and to follow
An easy and sustainable way for campuses to reduce waste is recycling bins. Therefore, option A is correct.
What are recycling bins ?Before being transported to recycling facilities, recyclables are stored in recycling bins. For usage inside and outside of residences, workplaces, and big public venues, recycling bins come in a variety of sizes.
The recycling bin's goal is to gather items that might have otherwise ended up in landfills. These items are frequently processed again to create new items. There are various items that can go in a recycling container. Papers, metal scraps, periodicals made of plastic, discarded gadgets, and a variety of other items are all acceptable.
The collection of used newspapers and magazines for recycling and their conversion into new paper products is an illustration of external recycling. Glass bottles and aluminum cans are two further examples of common items that are extensively recycled outside.
Thus, option A is correct.
To learn more about recycling bins, follow the link;
https://brainly.com/question/26216906
#SPJ12
Chlorine–35 has 17 protons. How many protons and neutrons does the isotope chlorine–36 have? 19 protons and 17 neutrons 17 protons and 18 neutrons 17 protons and 19 neutrons 18 protons and 17 neutrons
Answers
Answer:
17 protons
19 neutrons
Explanation:
Chlorine will always have the same amount of protons, and that would be 17 protons.
The atomic mass will change according to how many neutrons are present.
Cl - 35 is comprised of 17 protons and 18 neutrons.
We want to find Cl - 36:
We simply add 1 neutron. 18 + 1 = 19 neutrons.
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
identify the acid associated with each conjugate base. nh3 choose... I⁻ ___
SO4²⁻ ___
Cl⁻ ___ OH⁻ ___
F⁻ ___
a. HF
b. Water
c. Sulfuric acid d. Hydronium ion e. HCI f. НІ g. Bisulfate ion
Answers
The acid associated with \(NH_3\) is \(NH_4^+\), with I- is HI, with \(SO_4^{2-}\) is \(HSO_4^-\), with Cl- is HCl, with OH- is \(H_2O\), and with F- is HF.
1. NH3: It is a base that accepts a hydrogen ion (H+) from an acid. \(NH_3 + H^+ --> NH_4^+\). The acid associated with \(NH_3\) is \(NH_4^+\).
2. I-: is a base that accepts a hydrogen ion (H+) from an acid. \(I^- + H^+ --> HI\) . The acid associated with I- is HI.
3. \(SO_4^{2-}\) : is a base that accepts a hydrogen ion (H+) from an acid. \(SO_4^{2-} + H^+ --> HSO_4^-\). The acid associated with \(SO_4^{2-}\) is \(HSO_4^-\).
4. Cl-: is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when Cl- accepts a hydrogen ion (H+). \(Cl^- + H^+ --> HCl\). The acid associated with Cl- is HCl.
5. OH-: It is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when OH- accepts a hydrogen ion (H+). \(OH^- + H^+ --> H_2O\). The acid associated with OH- is \(H_2O\).
6. F-: It is a base that accepts a hydrogen ion (H+) from an acid. \(F^- + H^+ --> HF\). The acid associated with F- is HF.
To learn more about acid click here https://brainly.com/question/29796621
#SPJ11
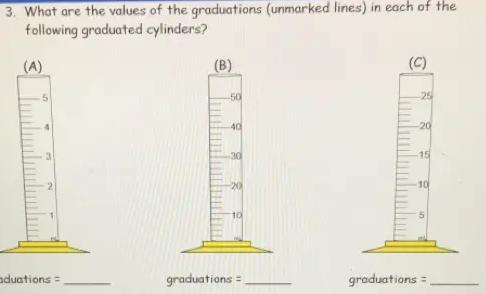
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Which of the following is true?
Select one:
O a. 212°F = 100°C
O b. All of the above
O c. -460°F = -273°C
O d. 32°F = 0°C
Answers
Answer:
b. all of the above
they are all correct
How much potassium nitrate could be dissolved into 2 L of water
Answers
Answer:
640 grams
Explanation:
look up Solubility table in wikipedia for potassium nitrate (KNO3)
32 grams of potassium nitrate (KNO3) water solubility at 20 degrees celsius (room temperature) can be dissolved in 100 milliliters (0.1 L) of water.
2 liters = 2000 milliliters
32 grams / 100 milliliters = x grams / 2000 milliliters
cross-multiply
100 * x = 32 * 2000
x = (32 * 2000) / 100
x ≈ 640 grams
chatgpt
a reaction produces 14.2 grams of a product. the theoretical yield of that product is 17.1 grams. which of the statements are true? select all that apply.
Answers
1. The actual yield is less than the theoretical yield. 2. The percent yield is approximately 83%. 3. There was a loss of product during the reaction. 4. The reaction did not go to completion. 5. The product is not pure.
To address your question regarding the reaction that produces 14.2 grams of a product with a theoretical yield of 17.1 grams, we need to consider the following terms:
1. Actual yield: This refers to the amount of product that is actually produced during a chemical reaction, which in this case is 14.2 grams.
2. Theoretical yield: This is the maximum amount of product that could be formed from a chemical reaction based on stoichiometry, and in this case, it is 17.1 grams.
Now, let's analyze the given statements to determine which are true:
A. The reaction has a 100% yield: This statement is false, as the actual yield (14.2 grams) is less than the theoretical yield (17.1 grams).
B. The reaction has a yield of less than 100%: This statement is true, as the actual yield is less than the theoretical yield.
C. The reaction has a yield of more than 100%: This statement is false, as the actual yield is less than the theoretical yield.
So, the correct answer is that the reaction has a yield of less than 100%.
To know more about theoritical yield visit:
https://brainly.com/question/1446190
#SPJ11
In a constant‑pressure calorimeter, 70.0 mL70.0 mL of 0.320 M Ba(OH)20.320 M Ba(OH)2 was added to 70.0 mL70.0 mL of 0.640 M HCl.0.640 M HCl. The reaction caused the temperature of the solution to rise from 23.00 ∘C23.00 ∘C to 27.36 ∘C.27.36 ∘C. If the solution has the same density and specific heat as water ( 1.00 g/mL1.00 g/mL and 4.184J/g⋅K,)4.184J/g⋅K,) respectively), what is ΔHΔH for this reaction (per mole H2OH2O produced)
Answers
Answer:
57.0kJ/mol is ΔH of the reaction
Explanation:
The reaction is:
1/2 Ba(OH)₂ + HCl → 1/2 BaCl₂ + H₂O + ΔH.
Where ΔH is the heat of reaction per mole of water.
Moles of water produced are equal to moles of HCl that are:
70.0mL = 0.070L * (0.640mol / L) = 0.0448moles HCl = Moles of water produced.
Now, heat produced is determined using coffee-cup calorimeter equation:
Q = m×ΔT×C
Where Q is heat released
m is mass of solution (70mL + 70mL = 140mL = 140g -Density of 1g/mL-)
ΔT is change in temperature (27.36°C - 23.00°C = 4.36°C)
And C is specific heat of the solution (4.184J/gK)
Replacing:
Q = 140g×4.36°C×4.184J/gK
Q = 2553.9J
This is the heat released when 0.0448 moles of water are produced, that means ΔH is:
2553.9J / 0.0448moles
ΔH = 57000J/mol =
57.0kJ/mol is ΔH of the reaction