How might the jetstream affect the winter in your town this winter explain your answer
Answers
The jetstream is a high-altitude, fast-moving air current that can impact weather patterns across large areas.
In the winter, changes in the jetstream can affect the amount and type of precipitation, as well as the temperature. For example, if the jetstream shifts southward, bringing colder air from the Arctic, your town may experience colder than average temperatures and more snowfall.
Alternatively, if the jetstream stays north, your town may experience milder temperatures and less precipitation. Overall, the jetstream can have a significant impact on the winter weather in your town, and it's important to keep an eye on its movements to prepare for any potential weather changes.
To know more about air current click on below link:
https://brainly.com/question/13653784#
#SPJ11
Related Questions
Can someone please help me

Answers
Answer:
white
Explanation:
A student started with 0.3295 g of copper. The mass of filter paper and copper (II) oxide product was found to be 0.5723 g, and that of the filter paper alone was 0.2568 g. Calculate the percent yield of copper (II) oxide.
Answers
The percent yield will be 95.75%
Percent yieldMass of copper = 0.3295 g
Mass of filter paper + copper (II) oxide = 0.5723
Mass of filter paper only = 0.2568
Mass of copper (II) oxide = 0.5723 - 0.2568 = 0.3155
Percent yield = yield/total x 100%
= 0.3155/0.3295 x 100% = 95.75%
More on percent yield can be found here: https://brainly.com/question/17042787
#SPJ1
Calculate the number of atoms in 18.34 grams of sulfur. Answers in scientific notation
PLZ HELP!!
Answers
Answer:
The answer is 1.834 * 10
Explanation:
guess the image time
hint its cringe

Answers
Answer:
what is going on their
Explanation:
yo thats kinda weird ummmmmm
how can i find wavelength in a wave?
Answers
Wavelength (L) is calculated using: L = gT²/2π, here g=9.8 m/s2 and T is wave period in seconds.
What is wavelength?Wavelength of a wave describes how long the wave is and the distance from the "crest" (top) of one wave to the crest of next wave is called wavelength. We can also measure from the "trough" (bottom) of one wave to trough of next wave and get the same value for the wavelength.
We measure wavelength in following ways:
Use photometer to measure the energy of wave.
Convert energy into joules (J).
Divide energy by Planck's constant, 6.626 x 10⁻³⁴, to get the frequency of wave.
Divide speed of light, ~300,000,000 m/s, by frequency to get wavelength.
To know more about wavelength, refer
https://brainly.com/question/10750459
#SPJ9
what are the two major components of the climate
Answers
Answer:
Water and carbon. They are important for climate, and they are important for life.
Which image shows a cumulonimbus cloud
1
2
3
4
Answers
Answer:
2
Explanation:
Answer:
2
Explanation:
i took the test on edg 2022 good luck on you test :)
What are the coefficients when this equation is balanced explain
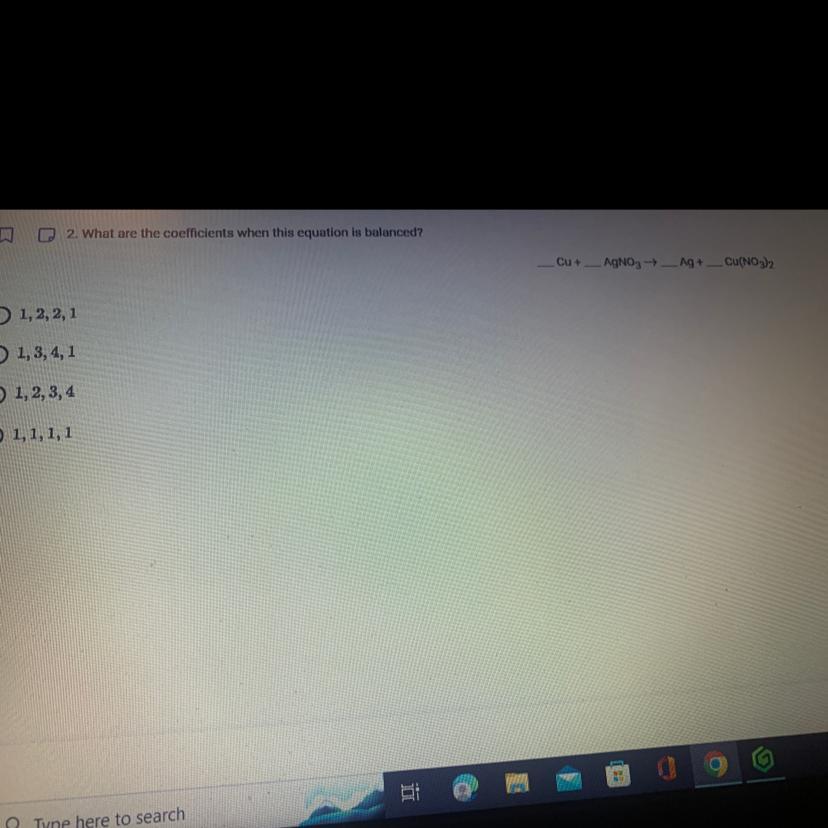
Answers
The balanced equation will be:
Cu + 2 AgNO3 ----> 2Ag + Cu(NO3)2
The balancing of equation is done to satisfy the law of conservation of mass. This law states - "mass can neither be created, nor be destroyed". Thus, the mass of the element in the equation cannot be created or destroyed, it is just shifted.
The answer is 1,2,2,1; the first option.
PLEASE HELP ME QUICK 30 POINTS RIGHT ANSWERS ONLY
Which of these compounds is likely to be bitter and have a ph above 7?

Answers
Answer:
NH₃ is bitter and has a pH above 7
Acids and Bases:Compounds that have a pH above 7 are known as bases. Properties of bases include:
taste bitterneutralise acidsturn red litmus blueCompounds that have a pH below 7 are known as acids. Properties of acids include:
taste sourneutralise basesturn blue litmus redThe first three compounds are acids. Their names are:
Phosphoric acid (H₃PO₄)
Nitric acid (HNO₃)
Hypochlorous acid (HClO)
The fourth compound is a ammonia (NH₃) which is a common base and therefore has a pH above 7, and tastes bitter.
HCN + Na0H = H,O + NaCN
In the reaction shown above, the
is the Arrhenius acid.
is the Arrhenius base, and the
NaOH; HCN
HCN; NaOH
H20; NaCN
NaCN; H20
Answers
Answer:
Explanation:
?
The Arrhenius acid is Hydrogen cynanide and the Arrhenius base is Sodium hydroxide (NaOH). The correct option is the second option.
Arrhenius acids & basesFrom the question, we are to determine which is the Arrhenius acid and base in the given equation.
From the Arrhenius definition of acids and bases
An acid is a substance that produces hydrogen ions (H⁺) in an aqueous solution
and
A base is a substance that produces hydroxide ions (OH⁻) in an aqueous solution
Please see the attached document for the complete step by step solution.
Learn more on Arrhenius acids & bases here: https://brainly.com/question/13603126
What mass of chromium could be deposited by electrolysis of an aqueous solution of Cr2(SO4)3 for 155 min using a constant current of 10.0 A
Answers
The mass of chromium that could be deposited by electrolysis of an aqueous solution of Cr2(SO4)3 for 155 min using a constant current of 10.0 A is approximately X grams.
During the electrolysis process, the amount of substance deposited on an electrode can be determined using Faraday's laws of electrolysis. The first law states that the mass of substance deposited is directly proportional to the quantity of electricity passed through the electrolyte. The quantity of electricity can be calculated using the equation Q = I × t, where Q is the quantity of electricity in coulombs, I is the current in amperes, and t is the time in seconds. Since the current is given as 10.0 A and the time is 155 min (or 9300 s), we can calculate Q as Q = 10.0 A × 9300 s.
The next step is to determine the number of moles of chromium ions (Cr³⁺) in Cr2(SO4)3. Cr2(SO4)3 contains 2 moles of chromium ions per formula unit. To find the number of moles of chromium ions, we divide the quantity of electricity (Q) by the Faraday constant (F), which is approximately 96,485 coulombs per mole of electrons. Thus, the number of moles of chromium ions is Q / F.
Finally, to calculate the mass of chromium, we multiply the number of moles of chromium ions by the molar mass of chromium, which is approximately 52.0 g/mol. Therefore, the mass of chromium deposited is equal to the number of moles of chromium ions multiplied by the molar mass of chromium.
To learn more about electrolysis, click here:
brainly.com/question/33301115
#SPJ11
Freezing point is considered a _________ physical property because ____________.
Question 6 options:
Extensive, it does not depend on how much matter is present.
Intensive, it does depend on how much matter is present.
Intensive, it does not depend on how much matter is present.
Extensive, it does depend on how much matter is present.
Answers
Answer:
Intensive, it does not depend on how much matter is present
so like.. no mater how much water you are freezing.. the freezing point will always be the same
explain heat of reaction in your own words
Answers
The change in the enthalpy of a chemical reaction that takes place under constant pressure is known as the Heat of Reaction.
What do you mean by the heat reaction ?The quantity of heat that has to be supplied or withdrawn during a chemical reaction in order to maintain the same temperature for all the reactants is known as the heat of reaction, also known as the enthalpy of reaction.
It is a thermodynamic unit of measurement that may be used to determine how much energy is released or created per mole during a process.
Thus, the change in the enthalpy of a chemical reaction that comes at a constant pressure is called as heat of reaction.
To learn more about the heat of reaction, follow the link;
https://brainly.com/question/30464598
#SPJ1
The most common isotope of uranium, 23892U, has atomic mass 238.050783 u.Calculate the mass defect.Calculate the binding energy.Calculate the binding energy per nucleon.
Answers
The mass defect uranium isotope is 0.342 u. The Binding energy is 3.08 x 10^10 J/mol and binding energy per nucleon is 1.29 x 10^-11 J/nucleon.
The mass defect is the difference between the mass of a nucleus and the sum of the masses of its constituent protons and neutrons. In this case, the mass defect can be calculated as:
mass defect = (238.050783 u - 92 x 1.007825 u - 146 x 1.008665 u) = 0.342 u
The binding energy is the energy required to separate a nucleus into its constituent protons and neutrons. It can be calculated from the mass defect using Einstein's famous equation E=mc^2, where c is the speed of light:
binding energy = (0.342 u)(1.66054 x 10^-27 kg/u)(2.998 x 10^8 m/s)^2 = 3.08 x 10^10 J/mol
The binding energy per nucleon is the binding energy divided by the number of nucleons in the nucleus. For uranium-238, there are 238 nucleons, so the binding energy per nucleon is:
binding energy per nucleon = (3.08 x 10^10 J/mol)/(238 nucleons) = 1.29 x 10^-11 J/nucleon
To learn more about binding energy; https://brainly.com/question/23020604
#SPJ11
Pls help me with this problem

Answers
Answer:
B
Explanation:
The triple point is the temperature and pressure in which the three states or phases of a matter coexist with one another.
For example, the triple point of pure water is 0.01°C and 4.5mmHg
If a system has 325 kcal
of work done to it, and releases 5.00×102 kJ
of heat into its surroundings, what is the change in internal energy (Δ or Δ)
of the system?
Answers
Internal energy of the system is 1278 kJ.
What is Internal Energy?
It refers to the intangible microscopic energy at the atomic and molecular scales, which is distinct in scale from the macroscopic organised energy associated with moving objects. For instance, a glass of water on a table at room temperature appears to have no apparent energy, either potential or kinetic.
∆U = w + q
U = modification of internal energy =?
W = work equals +425 kcal (positive sign because work is done on the system)
lthough I'm not sure you intended to use separate units (kcal and kJ), I'll presume you did so in order to solve the problem. Don't convert them if they should be the same, such as in the case of kJ.
425 kcal plus 4.184 kJ per kcal equals 1778 kJ of labor, or w.
∆U = +1778 + (-500 kJ) (-500 kJ)
∆U = +1278 kJ
Learn more about Internal Energy from given link
https://brainly.com/question/28302880
#SPJ1
Why are models used to represent atoms?
O A. Atoms are too small to see.
O B. Models are completely accurate.
O C. Models explain new evidence.
O D. Models never change over time.
Answers
please help me
16 1 point What is the decay rate of a sample of Oxygen-21 if the sample has 8.31x1017 atoms and a decay constant of 0.203/s? 4.09x1018Bq 1.69x10¹7Bq 0.203Bq 2.44x10-1⁹Bq Previous
Answers
decay rate of approximately 1.69x10^17 Bq (becquerels),
The decay rate of a radioactive sample is determined by the number of radioactive atoms present and the decay constant, which represents the probability of decay per unit of time.
To calculate the decay rate, we multiply the number of atoms in the sample by the decay constant. In this case, the sample has 8.31x10^17 atoms and a decay constant of 0.203/s. Multiplying these values gives a decay rate of approximately 1.69x10^17 Bq (becquerels), which represents the number of decays per second in the sample.
Learn more about Oxygen here : brainly.com/question/13905823
#SPJ11
How do we determine the volume of an irreguler shaped object?
Answers
Answer:
You can measure the displacement when the object is placed in a certain volume of water.
In the reaction of an alkyl bromide with sodium iodide in acetone, why would the resulting alkyl iodide be attacked by a bromide ion?
Answers
In the reaction of an alkyl bromide with sodium iodide in acetone, the resulting alkyl iodide may be attacked by a bromide ion due to a possible nucleophilic substitution reaction. During the initial reaction, the sodium iodide reacts with the alkyl bromide to form an alkyl iodide and sodium bromide.
However, if there is excess alkyl bromide present, the resulting alkyl iodide may undergo a second substitution reaction with the excess alkyl bromide acting as the nucleophile. This can occur because the alkyl iodide is still reactive and can be attacked by the bromide ion, which is also present in the reaction mixture. The resulting product would be a mixed alkyl halide containing both iodine and bromine.
Find out more about alkyl bromide
brainly.com/question/31412665
#SPJ11
Hypothesis: How easily for you think the following substances are fermented by yeast?
Answers
Yeast is a type of fungus that can ferment certain substances, meaning it breaks down sugars and converts them into alcohol and carbon dioxide. The ease with which a substance is fermented by yeast depends on a few factors, including the type of yeast being used and the composition of the substance itself.
Generally speaking, substances that contain a high amount of simple sugars are more easily fermented by yeast. This is because yeast is able to quickly and efficiently break down these sugars into alcohol and carbon dioxide. Examples of substances that are easily fermented by yeast include fruit juices, honey, and molasses.
On the other hand, substances that are more complex or contain less sugar may be more difficult for yeast to ferment. For example, yeast may have a harder time breaking down starches, such as those found in grains, without additional processing steps.
It's worth noting that different strains of yeast may also have varying levels of ability to ferment certain substances. Some strains may be better suited for fermenting certain types of beer or wine, for example, while others may be more effective at fermenting bread dough.
Overall, the ease with which a substance is fermented by yeast depends on a variety of factors, and may require some trial and error to determine the best approach for a particular substance.
Learn more about simple sugars here:
brainly.com/question/984360
#SPJ11
box a has a mass of 45.0kg and box b has a mass of 60.0kg. what is the tension on box a if the acceleration of the system is 1.40m/s2 clockwise? 378n 504n 1180n 882n
Answers
The tension acting on the box is 504 N.
Mass of the box A, m₁ = 45 kg
Mass of the box B, m₂ = 60 kg
Acceleration of the system, a = 1.4 m/s²
From, the figure, the forces acting on the blocks can be written as,
m₂g - T = m₂a ------eqn 1
T - m₁g = m₁a ------eqn 2
The magnitude of tension can be calculated by solving any of these equations. So, considering the first equation,
m₂g - T = m₂a
Therefore, the tension acting on the box,
T = m₂g -m₂a
T = m₂(g - a)
Applying the values of m₂, g and a,
T = 60 x (9.8 - 1.4)
T = 60 x 8.4
T = 504 N
To learn more about tension, click:
https://brainly.com/question/14429613
#SPJ1
PLEASE HELP ME QUICK RIGHT ANSWERS ONLY 40 POINTS :)
What does point f on the phase diagram represent?

Answers
The point F on the phase diagram represents normal melting point. Therefore, the correct option is option A.
A phase diagram is a sort of figure that is used throughout physical chemistry, technology, mineralogy, or materials science to demonstrate the circumstances (pressure, temperature, quantity, etc.) under which thermodynamically different phases (such solid, liquid, or gaseous states) arise or coexist at equilibrium. Lines of equilibrium, also known as phase boundaries, or circumstances under which different phases may remain at equilibrium, are typical elements of a phase diagram. The point F on the phase diagram represents normal melting point.
Therefore, the correct option is option A.
To know more about phase diagram, here:
https://brainly.com/question/31251128
#SPJ1
Two chemical reactions are shown. Which statements is true?

Answers
Answer:
The answer is b
Explanation:
they both are decomposition
Please can someone help I will mark brainiest!

Answers
Answer:
2Cl- ⇒ Cl ↓2+ 2e
Explanation: sorry if this is not what you were looking for.
In the laboratory, a general chemistry student measured the pH of a 0.494 M aqueous solution of isoquinoline, C9H7N to be 9.559. Use the information she obtained to determine the Kb for this base.
Answers
By plugging in the values obtained in steps 6 and 7, we can calculate the Kb for isoquinoline.
To determine the Kb (base dissociation constant) for the base isoquinoline (C9H7N) based on the measured pH, we can use the following steps:
1. Calculate the pOH of the solution by subtracting the measured pH from 14: pOH = 14 - pH = 14 - 9.559 = 4.441.
2. Convert the pOH value to OH- concentration using the equation: pOH = -log[OH-]. Rearranging the equation gives: [OH-] = 10^(-pOH) = 10^(-4.441).
3. Since isoquinoline (C9H7N) acts as a base by accepting a proton (H+) to form its conjugate acid, we can assume that [OH-] is equal to the concentration of the isoquinoline in the solution, [C9H7N].
4. Set up the equilibrium expression for the reaction of isoquinoline with water: C9H7N + H2O ⇌ C9H7NH+ + OH-.
5. Use the stoichiometry of the balanced equation to express [OH-] in terms of [C9H7NH+]: [OH-] = [C9H7NH+].
6. Substitute the concentration [OH-] obtained in step 2 into the equilibrium expression: [C9H7N] = [OH-] = 10^(-4.441).
7. Calculate the concentration of C9H7NH+ by subtracting [C9H7N] from the initial concentration: [C9H7NH+] = 0.494 M - 10^(-4.441) M.
8. Finally, calculate the Kb using the equation: Kb = [C9H7NH+][OH-] / [C9H7N].
To know more about isoquinoline visit:
https://brainly.com/question/7428881
#SPJ11
lead (pb) is a group 14 element which has 4 valence electrons. how many bonds does this element usually form?
Answers
Lead (Pb), being a group 14 element with 4 valence electrons, usually forms 2 bonds.
Valence electrons are the outermost electrons of an atom that participate in chemical bonding. In the case of lead (Pb), it has 4 valence electrons, which means it can either gain or lose 4 electrons to achieve a stable electron configuration or share its electrons with other atoms to form chemical bonds. However, lead typically forms covalent bonds, which means it shares its electrons with other atoms to complete its octet configuration. Since lead has 4 valence electrons, it can form 2 covalent bonds with other elements that have a tendency to gain or share electrons.
Therefore, lead (Pb) usually forms 2 bonds due to the presence of 4 valence electrons, which can be shared with other elements to form covalent bonds.
learn more about valence electrons
https://brainly.com/question/371590
#SPJ11
What is the name of this hydrocarbon? A skeletal model starts with C H 3 at the left, goes down to a valley, up to a peak, and then down again. The valley and the peak have C H 3; there is also a C H 3 on a line straight down from the end of the centerline of carbons.
Answers
The name of the hydrocarbon can be determined using the skeletal structure of the molecule. The hydrocarbon with the given skeletal structure is isobutane.
Since the skeletal structure starts with CH3 at the left, goes down to a valley, up to a peak, and then down again and there is also a CH3 on a line straight down from the end of the centerline of carbons, we can conclude that the molecule is isobutane. Isobutane is a branched-chain hydrocarbon with the molecular formula C4H10. It is an alkane with four carbon atoms and ten hydrogen atoms. Isobutane is commonly used as a fuel for stoves, lighters, and torches because it is a highly flammable gas that burns cleanly and efficiently.
The hydrocarbon with the given skeletal structure is isobutane.
To know more about molecule visit:
brainly.com/question/32298217
#SPJ11
6Na + Fez0g -> 3NazO + 2Fe
If you are provided 200g of sodium and 250 grams of iron(Ill) oxide, how much of excess reagent is left?
Answers
The amount of excess reagent that will remain would be 11.76 g.
Stoichiometric problemTo determine the excess reagent in the reaction, we need to first determine which reactant is limiting and which reactant is in excess.
The balanced chemical equation for the reaction is:
6Na + Fe2O3 -> 3Na2O + 2Fe
The molar mass of Na is 23 g/mol, and the molar mass of Fe2O3 is 159.69 g/mol (2 x 55.85 g/mol for Fe + 3 x 16 g/mol for O).
Using the given masses, we can calculate the number of moles of each reactant:
Number of moles of Na = 200 g / 23 g/mol = 8.70 molNumber of moles of Fe2O3 = 250 g / 159.69 g/mol = 1.57 molAccording to the balanced chemical equation, 6 moles of Na react with 1 mole of Fe2O3. Therefore, the number of moles of Na required to react with 1.57 mol of Fe2O3 is:
(1.57 mol Fe2O3) x (6 mol Na/1 mol Fe2O3) = 9.42 mol Na
Since we only have 8.70 mol of Na available, it is the limiting reagent. This means that Fe2O3 is in excess.
To determine the amount of excess Fe2O3, we need to calculate how much Fe2O3 is required to react with 8.70 mol of Na:
(8.70 mol Na) x (1 mol Fe2O3/6 mol Na) x (159.69 g/mol Fe2O3) = 238.24 g Fe2O3
Since we only have 250 g of Fe2O3, the amount of excess Fe2O3 is:
250 g - 238.24 g = 11.76 g
Therefore, the amount of excess Fe2O3 left after the reaction is 11.76 g.
More on stoichiometry can be found here: https://brainly.com/question/29775083
#SPJ1
giving brainly if correct and detailed!

Answers
Answer:
put particles far apart and arrows pointing