Answers
Answer:
3291.84 cm
Explanation:
you would multiply the amount of yards you have by 91.44, since that's how many centimeters there are in 1 yd.
Answer: it’s D
Explanation:
Related Questions
what will the volume be of a solution created using 120 ml of 4.50 m stock solution if the final molarity needs to be 2.00 m?
Answers
The volume of the solution created using 120 mL of 4.50 M stock solution with a final molarity of 2.00 M is 270 mL.
M1V1 = M2V2
Substituting in the given values, we get:
(4.50 M)(120 mL) = (2.00 M)(V2)
Simplifying and solving for V2, we get:
V2 = (4.50 M)(120 mL) / (2.00 M)
V2 = 270 mL
Molarity is a unit of concentration commonly used in chemistry. It is defined as the number of moles of a solute dissolved in one liter of solution. In other words, molarity is a measure of how much solute is present in a given volume of solution.
Moles are used to measure the amount of a substance in a sample. One mole of a substance is defined as the amount of that substance that contains the same number of particles as there are atoms in 12 grams of carbon-12. For example, one mole of water contains 6.02 x \(10^{23\) water molecules.
To know more about Molarity refer to-
brainly.com/question/2817451
#SPJ4
water at 20 degrees celsius is placed on a stove. as the water is heated, thermal energy is absorbed by the water. if the water is heated to a maximum temperature of 80 degrees celsius, what happens as a result of the increased temperature?
Answers
Temperature affect the kinetic energy of water molecules.
Does the temperature increase affect the molecules of water?An increase in temperature does affect the molecules of water. As the temperature of water increases, the molecules gain kinetic energy and move faster, which causes the bonds between the water molecules to weaken and the water molecules to spread out, resulting in an increase in volume.
The increase in temperature affects the kinetic energy and movement of water molecules, which in turn affects the physical state of water and the way it interacts with its environment.
Learn more about temperature:https://brainly.com/question/11464844
#SPJ1
Which of the following are important properties of RNA polymerase from E. coli?
It uses a single strand of dsDNA to direct RNA synthesis.
It is composed of five different subunits.
It has a molecular weight of about 500 Da.
It reads the DNA template from its 3' end to its 5' end during RNA synthesis.
Answers
The important properties of RNA polymerase from E. coli are It reads the DNA template from its 3' end to its 5' end during RNA synthesis and It uses a single strand of dsDNA to direct RNA synthesis. It is composed of five different subunits. SO, Option D, A and B are correct.
It is a multisubunit enzyme that contains many functional regions that are critical for the synthesis of RNA from a DNA template.The RNA polymerase of E. coli is a complex enzyme that has a number of important properties. The RNA polymerase is composed of five different subunits that are arranged in a holoenzyme configuration.
This holoenzyme is responsible for the recognition of promoter sequences on the DNA template and the subsequent initiation of RNA synthesis. RNA polymerase from E. coli reads the DNA template from its 3' end to its 5' end during RNA synthesis. This is in contrast to DNA polymerase, which reads the DNA template from its 5' end to its 3' end during DNA replication.
RNA polymerase from E. coli uses a single strand of dsDNA to direct RNA synthesis. The enzyme recognizes the template strand and reads it in the 3' to 5' direction, synthesizing the RNA strand in the 5' to 3' direction. This process is called transcription.
Therefore, Option A,B, and D are correct.
Learn more about RNA polymerase -
brainly.com/question/31141023
#SPJ11
Draw Molecular diagrams of solid, liquid, gas and plasma phases of matter.
Answers
Answer:
Explanation:
Hope this helped!

how many moles of oxygen atoms are in one mole of the molecule Ca5(PO4)3OH? could you also show work
Answers
Answer:
13 moles of oxygen
Explanation:
A mole of a substance is the unit of measuring the number of particles within a chemical substance.
The given compound is:
Ca₅ (PO₄)₃OH
This is a mole of Ca₅ (PO₄)₃OH
In this compound we have:
5 moles of Ca
3 mole of P
13 mole of O
1 mole of H
So,
In 1 mole of Ca₅ (PO₄)₃OH, we have 13 moles of oxygen
Finally we can calculate the final volume of the solutioWhat final volume will produce a solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3?
_______ L solutionn.
Answers
The final volume of solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3 219 L solution.
Thus, M, or moles/liter, is the sign for molarity. Additionally, chemists utilize square brackets to denote a reference to a substance's molarity. The molarity of the silver ion in solution, for instance, is shown by the formula [Ag+].
The most straightforward to calculate with but the most challenging to create in the lab are solution concentrations stated in molarity. When addressing chemical reactions in which a solute is a product or a reactant, such concentration units are helpful.
Then, in order to translate numbers in moles to amounts in grams, molar mass can be employed as a conversion factor. The terms "mol" and "L" in this statement stand for moles of solute.
Thus, The final volume of solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3 219 L solution.
Learn more about Volume, refer to the link:
https://brainly.com/question/28058531
#SPJ4
I'm lyrics texting my crush and want to know what song i should do
Answers
Answer: mine by bazzie
jus' gonna tell you how i feel (look)
you so focking precious when you smile (heh,yeah)
hit it from the back and drive you wild, oh yeah
girl/boy, lose myself in those eyess
i just had to let you know you're mineee
how have humans effected climate change?
Answers
The anthropogenic activities such as the release of carbon dioxide has contributed to climate change.
What is climate change?Large amounts of carbon dioxide are released into the atmosphere via the combustion of fossil fuels like coal, oil, and natural gas for energy production, transportation, and industrial activities. A greenhouse gas called carbon dioxide traps heat in the atmosphere of the Earth, causing the greenhouse effect and global warming.
Large forested areas have been lost as a result of deforestation, which is mostly caused by logging, urbanization, and agricultural development. As part of their photosynthesis, trees serve as carbon sinks by absorbing carbon dioxide.
Learn more about climate change:https://brainly.com/question/31604908
#SPJ1
Which example is Not an example of Chemical Potential energy. The hamburger, The Spring, The battery, the flask
Answers
Answer:
Hamburger
Explanation:
What is used to determine the number of each atom in an ionic formula
Answers
Answer:
The charge carried by each ion (oxidation state of each atom)
Explanation:
If we have an ionic compound and we want to write its formula, we must first know the magnitude of charge on each ion (shown as oxidation state of the atoms involved) because the magnitude of charge on each ion is eventually crisscrossed and gives the subscript (number of atoms) for each atom in the formula.
For instance, let us write the formula of calcium bromide. Ca has a charge of +2 while Br has a charge of -1. If we exchange the charges and ignore the signs such that the crisscrossed charges form subscripts we can now write; \(CaBr_{2}\).
(3) With two substances that are exactly the same size the mass may be different due to the type of:HELP!!
Answers
When two substances have the same size but different masses, it is usually due to differences in their chemical composition or density.
Chemical Composition: Different substances have different types and arrangements of atoms or molecules. The atomic or molecular mass of a substance is determined by the sum of the masses of its constituent particles. Therefore, substances with different chemical compositions will have different masses, even if they have the same size or volume.
Density: Density is a measure of how much mass is packed into a given volume. Substances with different densities will have different masses, even if they have the same size. Density is influenced by factors such as the arrangement and packing of particles, intermolecular forces, and atomic or molecular mass.
It's important to note that the size and mass of a substance are independent of each other. Two substances can have the same size or volume but different masses due to their composition and density.
Learn more about density here:
https://brainly.com/question/29775886
#SPJ11
newton's 3rd law: for every_____there is an_____and_____reaction
Answers
There are total three laws of newtons, first law of newtons, second law of newton and third law of newton. Therefore, for every action there is an equal and opposite reaction.
What is newton's third law?Newton's first law is also called law of inertia. An object at rest remains at rest, and an object in motion remains in motion at constant speed and in a straight line unless acted on by an unbalanced force.
Third law of newton states that for every action there is an equal and opposite reaction.
Therefore, for every action there is an equal and opposite reaction.
To know more about newton's law, here:
https://brainly.com/question/29768600
#SPJ1
What is the name given to the process shown in the diagram
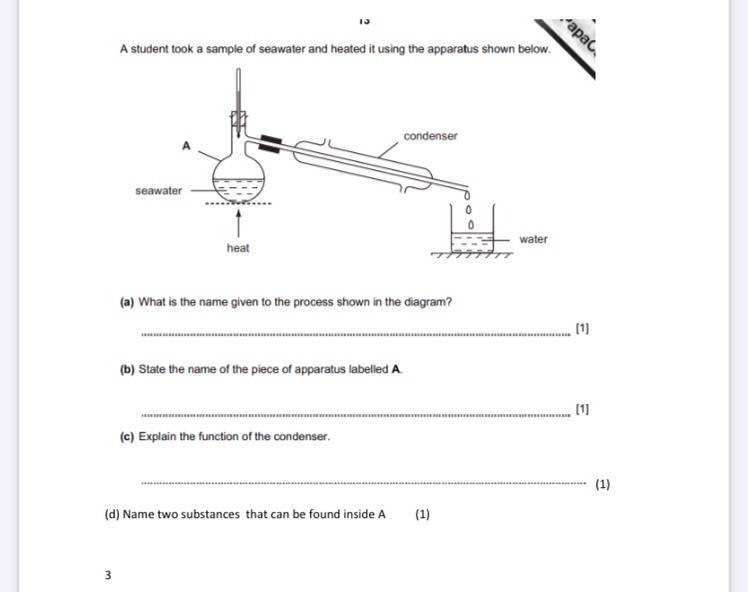
Answers
which type of chemical penetrates the eye deeper and continues to burn longer?
Answers
Strong acids tend to penetrate the eye deeper and continue to burn longer compared to strong bases. The corrosive nature of strong acids allows them to cause severe damage upon contact with the eye, leading to a longer-lasting burning sensation.
Strong acids, such as sulfuric acid (H2SO4) and hydrochloric acid (HCl), have the ability to donate protons (H+) in solution. When they come into contact with the eye, they can cause chemical burns due to their corrosive properties. The acidic nature of these compounds enables them to penetrate the tissues of the eye more deeply, leading to a prolonged burning sensation.
In contrast, strong bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH), also possess corrosive properties but typically do not penetrate as deeply as strong acids. Bases tend to react with the outer layers of the eye, resulting in a relatively shorter duration of burning sensation.
It is important to note that both strong acids and strong bases can cause significant harm to the eye, and immediate medical attention is necessary in case of exposure.
Learn more about corrosive here: brainly.com/question/31313074
#SPJ11
Strong acids tend to penetrate the eye deeper and continue to burn longer compared to other types of chemicals.
What is a strong acid?
A strong acid is an acid that completely dissociates or ionizes in water, releasing a high concentration of hydrogen ions (H⁺). This dissociation process is essentially irreversible, meaning that nearly all of the acid molecules break apart into ions when dissolved in water. As a result, strong acids are highly acidic and have a low pH value.
Strong acids are typically more corrosive and can penetrate the eye deeper, causing more severe damage and continuing to burn for a longer duration compared to weak acids or other chemicals.
When a strong acid comes into contact with the eye, it can cause immediate and severe damage due to its ability to donate protons (H+) readily and fully dissociate in water. Strong acids such as sulfuric acid (H₂SO₄), hydrochloric acid (HCl), and nitric acid (HNO₃) have the potential to cause extensive tissue damage, leading to long-lasting burning sensations and severe injury to the eye.
It is crucial to exercise extreme caution when handling or being exposed to strong acids, and immediate medical attention should be sought if an eye injury occurs.
To learn more about strong acid from the given link
brainly.com/question/30782679
#SPJ4
How does the rate of today's warming compare to previous episodes of
rapid climate change on Earth?
A. Today's climate warming is about as fast as the temperature swings that have
happened in Earth's past.
B. Past changes in the climate have been faster than the changes we're seeing today.
C. Today, the Earth's climate is changing much faster than it has changed in the past.
Answers
Answer:
B. Past changes in the climate have been faster than the changes we're seeing today
Explanation:
The Earth is warming abnormally quickly . Over the past century it has warmed roughly 10 times faster than the average increase in temperature after each ice age .
so the answer is B.Past changes in the climate have been faster than the changes we're seeing today.
what is known as the "father of chemistry" ?
Answers
Answer:
It's would be Jöns Jacob Berzelius
Answer:
Antoine Laurent Lavoisier is consider as the Father of Chemistry. He was a meticulous experimenter, he revolutionized chemistry.
Q4 This question relates the combustion reactions of acetylene, hydrogen and ethane. (a) Express the stoichiometric ecpigtions for the combustion reactions of acetylene, hydrogen and ethane with their respective standard heats of combustion obtained from physical property table. (b) Verify the standard heat of combustion of acetylene in Q4(a) by using heat of formation method. (c) The equation below shows the acerylene hydrogenation reaction: C2H2(g)+2H2(g)→C2H6(g) (i) Compute the standard heat of acetylcne hydrogenation reaction using tabulated heats of formation and heats of combustion. (ii) Verify the answer in Q4(e)(1) by using Hess's Law.
Answers
Stoichiometric equations for the combustion reactions ΔHf° (C2H2) = (2 x (-393.5)) + (-285.8) - (-1299.5) = +226.7 kJ mol-1(c) Acetylene hydrogenation reaction
Acetylene combustion reaction:C2H2 (g) + (5/2) O2 (g) → 2 CO2 (g) + H2O (l) ΔHc° = -1299.5 kJ mol-1 Hydrogen combustion reaction:2H2 (g) + O2 (g) → 2 H2O (l) ΔHc° = - 483.7 kJ mol-1Ethane combustion reaction:C2H6 (g) + (7/2) O2 (g) → 2 CO2 (g) + 3 H2O (l) ΔHc° = - 1560 kJ mol-1(b) Heat of formation method for verifying the standard heat of combustion of acetylene: The standard heat of combustion of acetylene from the heat of formation method is:ΔHc° (C2H2) = 2 ΔHf° (CO2) + ΔHf° (H2O) - 2 ΔHf° (C2H2) = -1299.5 kJ mol-1ΔHf° (CO2) = -393.5 kJ mol-1ΔHf° (H2O) = -285.8 kJ mol-1.
For verifying the answer in Q4(e)(1) using Hess's Law, we need to convert acetylene hydrogenation reaction into a combination of other reactions:Reaction 1:C2H2 (g) + (2.5) O2 (g) → 2 CO2 (g) + H2O (l) ΔH1 = -1299.5 kJ mol-1Reaction 2:2 CO2 (g) + 2.5 H2 (g) → C2H6 (g) + 5 O2 (g) ΔH2 = +1560 kJ mol-1After multiplying and adding the above equations, we get the required reaction as:C2H2 (g) + 2 H2 (g) → C2H6 (g) ΔH = -396.1 kJ mol-1.
To know more about reactions visit:
https://brainly.com/question/16737295
#SPJ11
What period is the atom in?
What group is the atom in?
What is the name of this atom?
What other atom would have similar properties
to the atom?
Would this atom be malleable or brittle?

Answers
It is in group 17
It is a chlorine atom because it has 17 electrons which means the atomic number is 17
In what way are chemicals a part of our everyday lives?
A. Chemicals are used only to make food.
B. We are dependent on chemicals because we have been using
them for centuries.
C. Chemicals are used only in products that are shipped overseas.
D. Naturally occurring and man-made chemicals are in everything
around us.
Answers
Answer:
it's D
Explanation:
I hope this helps you
Let G and H be groups. Prove if φ(g) = eH for all g ∈ G, the map φ: G to H is a group homomorphism
Answers
φ(g1 * g2) = eH = φ(g1) * φ(g2).
This completes the proof that φ: G → H is a group homomorphism.
By showing that the map φ preserves the group operation, we have demonstrated that it is a group homomorphism.
To prove that φ: G → H is a group homomorphism, we need to show that it preserves the group operation. In other words, for any two elements g1 and g2 in G, φ(g1 * g2) = φ(g1) * φ(g2), where * denotes the group operation in G, and * denotes the group operation in H.
Given that φ(g) = eH for all g ∈ G, where eH is the identity element in H, we can start the proof as follows:
Let g1, g2 ∈ G. We want to show that φ(g1 * g2) = φ(g1) * φ(g2).
Since φ(g) = eH for all g ∈ G, we have φ(g1) = eH and φ(g2) = eH.
Now, consider the product g1 * g2 in G. Applying φ to both sides, we have:
φ(g1 * g2) = φ(g1) * φ(g2).
Substituting the values of φ(g1) and φ(g2), we get:
φ(g1 * g2) = eH * eH.
Since eH is the identity element in H, the product eH * eH is simply eH.
To know more about homomorphism
https://brainly.com/question/6111672
#SPJ11
do you expect a similar reaction between potassium metal and elemental bromine?
Answers
Yes, a similar reaction is expected between potassium metal and elemental bromine. Both potassium and bromine are highly reactive elements. When they come into contact with each other, they will undergo a redox reaction where potassium will lose one electron to bromine to form KBr.
The reaction is exothermic, and it occurs violently, producing a bright light and a lot of heat. It is highly recommended not to attempt this reaction outside of a laboratory setting, as it can be very dangerous and can cause serious injury.
To know more about reaction click this link -
brainly.com/question/28984750
#SPJ11
What is the basic mechanism that naturally creates freshwater within the hydrologic cycle? precipitation reverse osmosis evaporation runoff infiltration
Answers
The basic mechanism that naturally creates freshwater within the hydrologic cycle is through the processes of evaporation, precipitation, infiltration, and runoff.
1. Evaporation: Water from the Earth's surface (e.g., oceans, lakes, and rivers) is heated by the sun and turns into water vapor, rising into the atmosphere.
2. Condensation: The water vapor cools as it rises, condensing into clouds.
3. Precipitation: When the clouds become heavy enough, the water droplets combine and fall back to the Earth's surface as precipitation (e.g., rain, snow, or hail).
4. Infiltration: Precipitation that reaches the ground can infiltrate the soil, becoming part of the groundwater system.
5. Runoff: Precipitation that does not infiltrate the soil will flow over the land surface as runoff, eventually entering rivers, lakes, and oceans.
This continuous movement of water through the various stages is known as the hydrologic cycle. Note that reverse osmosis is not part of this natural process; it is a human-engineered method used for water purification.
More on hydrologic cycle: https://brainly.com/question/13334963
#SPJ11
Someone pls help me I will make you brain

Answers
Answer:
I would go with the first option. It shows how people having been releasing more and more carbon dioxide into the atmosphere.
Explanation:
What does it mean for a reaction to release energy?
A The activation energy of the reaction is positive.
B The relative potential energy of the reaction is positive.
C The relative potential energy of the reaction is negative.
D The activation energy of the reaction is negative.
Answers
In this case, the problem is providing information about the meaning of the release of energy in a chemical reaction and some choices are given yet the correct one is C, "the relative potential energy of the reaction is negative" according to the following:
Thermochemistry:One branch of chemistry is thermochemistry and is widely used for us to realize how much or what type of energy is involved in a chemical change. Thus, we define two types of reactions in this regard, endothermic and exothermic. The former, takes place when the system absorbs energy to carry out the reaction, so the relative potential energy turns out to be positive.
On the other hand, exothermic reactions take place when the system releases energy, so the relative potential energy turns out to be negative. In such a way, we conclude the answer is C "the relative potential energy of the reaction is negative" according to the aforementioned.
Learn more about endothermic and exothermic reactions: https://brainly.com/question/4345448
A lithium atom contains 3 protons , 4 neutrons and 3 electrons
Answers
Where is DNA found?
a. cell membrane
b. vacuole
c. chromosomes
d. Golgi body
Answers
Answer:
You get DNA from your cell membrane
Explanation:
Superfine 40 gauge copper wire has a diameter of only 0.080 mm and weighs only 44.5 g/km. Suppose it’s full of 40 gauge wire weighs 471. g Less after some wire is pulled off to wind a magnet. How could you calculate how much wire is used?Set the matter. But don’t do any of it. Just leave your answer as a math expression. Also be sure your answer includes all the correct unit symbols.Length of wire = ?

Answers
- To calculate the length of wire we can use a mathematical Rule of Three:
\(\text{length of wire=}\frac{wire\text{ weighs. 1km}}{44.5g}\)- So, in this case the length would be:
\(\begin{gathered} \text{length of wire=}\frac{\text{471g.1km}}{44.5g} \\ \text{length of wire=10.58km} \end{gathered}\)We can write the math expression as:
\(L=\frac{w.1\operatorname{km}}{44.5g}\)Here, L is the length of wire and w is the wire weighs.
If
Half life of an isotope is 12 days and it was assumed that the
person ate 400 Bq of isotope. Using the GI track model information,
calculate the number of transformations in Stomach
Answers
If half life of an isotope is 12 days, then there are about 820.42 transformations in the stomach after the person ate 400 Bq of the isotope.
Using the GI track model information, the number of transformations in Stomach can be calculated as follows :
We know that the half-life of an isotope is defined as the time taken for half of the radioactive atoms to decay.
The decay of the isotope can be represented by the following formula : N(t) = N0e^(-λt)
where:
N(t) = Number of atoms at time t
N0 = Initial number of atoms
λ = Decay constant
t = Time elapsed from the initial time t = 0
For a given isotope, the decay constant is related to the half-life as follows : λ = 0.693/T1/2
where : T1/2 = Half-life time of the isotope
Given that the half-life of the isotope is 12 days, we can calculate the decay constant as follows :
λ = 0.693/12 = 0.0577 day^(-1)
The number of transformations in the stomach can be calculated by using the following formula :
Activity = A0e^(-λt)
where : A0 = Initial activity of the isotope in Bq
λ = Decay constant
t = Time elapsed from the initial time t = 0
Activity = 400 Bq (Given)
Decay constant (λ) = 0.0577 day^(-1)
Time elapsed (t) = Time taken by the isotope to reach the stomach from the time of consumption = 0.17 days (Given by GI track model)
Therefore, the number of transformations in the stomach is :
Activity = A0e^(-λt)A0 = Activity/e^(-λt)A0 = 400 Bq/e^(-0.0577 × 0.17)A0 = 400 Bq/e^(-0.009809)A0 = 447.45 Bq
The number of transformations in the stomach can be calculated as follows :
Number of transformations = Activity decayed per unit time/Disintegration constant
Activity decayed per unit time = A0 - Activity after time elapsed
Activity decayed per unit time = 447.45 - 400 = 47.45 Bq
Disintegration constant = Decay constant = 0.0577 day^(-1)
Therefore, number of transformations = (447.45 - 400) Bq/0.0577 day^(-1)
Number of transformations = 820.42
This means that there are about 820.42 transformations in the stomach after the person ate 400 Bq of the isotope.
To learn more about half-life :
https://brainly.com/question/1160651
#SPJ11
FeBr3+Ba(OH)2——-Fe(OH)3+BaBr2
Answers
Answer:
Balanced Chemical Equation
2FeBr3 + 3Ba(OH)2 → 2Fe(OH)3 + 3BaBr2
Explanation:
Reaction Information
Ferric Bromide + Barium Hydroxide = Iron(III) Hydroxide + Barium Bromide
Reaction Type
Double Displacement (Metathesis)
Reactants
Ferric Bromide - FeBr3
FeBr3
Molar Mass of Br3Fe Oxidation State of Br3Fe
Barium Hydroxide - Ba(OH)2
Caustic Baryta Barium Hydroxide Lime Ba(OH)2 Barium Dihydroxide Hydrate
Molar Mass of BaH2O2 Oxidation State of BaH2O2
Products
Iron(III) Hydroxide - Fe(OH)3
Ferric Hydroxide Ferric Oxide Yellow
Molar Mass of FeH3O3 Oxidation State of FeH3O3
Barium Bromide - BaBr2
Molar Mass of BaBr2 Oxidation State of BaBr2
FeBr3 + Ba(OH)2 = Fe(OH)3 + BaBr2
Instructions
To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F.Ionic charges are not yet supported and will be ignored.Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will.Compound states [like (s) (aq) or (g)] are not required.You can use parenthesis () or brackets []i hope it's help you
Potassium-40 (40k) is a radioactive material that decays into argon-40 (40ar). the half-life of a sample of 40k is 1.3 billion years. rocks containing 40k have been around since the formation of the earth, and 40ar gas has been accumulating in those rocks since the earth formed. however, when rocks are heated by volcanic action, all the 40ar leaves the rock when the gases escape. create a scenario and discuss, based on your knowledge of half-life, the value of potassium-argon dating to geologists.
Answers
The Potassium-40 (40k) is a radioactive material that decays into argon-40 . the half-life of a sample of 40k is 1.3 billion years. the potassium - argon dating is the method that based on the radiometric dating.
The potassium - argon dating is the type of the geological method that is based on the radiometric dating. it detect the volcanic ash of rocks. the principles it is based on the is the radioactive disintegration. the half life of the sample is 1.3 billion years. the radioactive potassium isotope that is potassium 40 is in volcanic rocks. this will disintegrate at the known rate is inert gas that is argon 40.
The gas will be accumulated in the rock crystal. and determine several billions years. the potassium found in the micas, hornblendes and the feldspars.
To learn more about half life here
https://brainly.com/question/13947504
#SPJ4
