If an area has a very cold climate, it is most likely that the area
Answers
If an area has a very cold climate, it is most likely that the area experiences low temperatures throughout the year.
Cold climate regions are often characterized by sub-zero temperatures and limited precipitation, which can lead to dry and barren landscapes. These regions are typically found in the polar regions of the world, such as the Arctic and Antarctic, as well as in high-altitude mountain ranges.
The cold climate can have a significant impact on the environment, with many plants and animals adapted to survive in the harsh conditions. In cold climates, plants and animals often have adaptations that help them conserve heat and energy, such as thick fur coats, hibernation, or slow growth rates.
This means that the biodiversity in cold climate regions may be different than that found in more temperate regions.
Human communities that live in cold climate regions have also adapted to the extreme conditions, often relying on traditional techniques to survive. For example, the Inuit people of the Arctic have developed an intricate knowledge of the land and sea to hunt, fish, and gather food. They have also developed specialized tools and clothing to withstand the cold temperatures.
Overall, a cold climate can have a significant impact on the environment and the communities that rely on it. Understanding the unique challenges and adaptations of these regions is crucial for effective conservation and management.
To know more about cold climate, visit:
https://brainly.com/question/11673115#
#SPJ11
Related Questions
2.9g of a gas at 95°C occupied the same volume as 0.184g of dihydrogen at 17°C at the same pressure. What is the molar mass of the gas?
Answers
The molar mass of the gas at the same pressure is M = 40g/mol.
Equation :Let M, be the molar mass of the gas
The number of moles of the gas,
n= 2.9 / M
The volume of the gas,
V= P/nRT
V = 2.9R x 368 / MP
The number of moles of dihydrogen,
n = 0.184 / 2
n =0.092
The volume of dihydrogen gas,
v = 0.092×R×290 / P
At the same pressure P,
The volume of gas = volume of dihydrogen
0.092R×290= 2.9R×368 / M
M= 2.9×368 / 0.092×290
M = 40
Hence, the molar mass of the gas is 40g/mol.
To know more about dihydrogen :
https://brainly.com/question/16934482
#SPJ9
What are the unique characteristics of carbon, which give it the ability to form a vast array of chemical substances?
Answers
Answer:
Carbon has an exceptional ability to bind with a wide variety of other elements. Carbon makes four electrons available to form covalent chemical bonds, allowing carbon atoms to form multiple stable bonds with other small atoms, including hydrogen, oxygen, and nitrogen.
plz follow me
Answer:
carbon has an exceptional ability to blind with a wide variety of other elements. carbon make 4 electrons available to form covalent chemical bonds, allowing carbon atom
What does a scientist mean when he or she says an object is at rest?(1 point) Responses The object is not speeding up or slowing down. The object is not speeding up or slowing down.
The object has no forces acting on it.
The object has no forces acting
on it. The object has no attractive forces acting on it. The object has no attractive forces acting on it. The object is not moving relative to its surroundings.
Answers
Scientist mean when he or she says an object is at rest then the object is not moving relative to its surroundings
Velocity of the object is said to be uniform when its speed and direction does not change and the object at rest has uniform speed of zero all the time and does not even change the direction and when object is at rest position then the velocity is zero such an object will not change its state of motion that's why when he or she says an object is at rest the scientist says that the object is not moving relative to its surroundings
Know more about object
https://brainly.com/question/14362959
#SPJ1
Convert the following. Show your work for full credit.(2pts each: 16 pts)
1. 2 inches = _______________mm
2. 7 lb = _________________g
3. 3 ft.= _________________cm
4. 16 gallons = _________________L
5. 457° C = ____________________° F
6. 104° F = ____________________° C
7. 4 inches = __________________ cm
8. 57kg = ___________________lbs.

Answers
Answer:
50.8 mm
3175.15 g
91.44 cm
0.5666 L
854.6 °F
Explanation:
1) 2 inches ... mm
1 inch = 25.4 mm
2 inches will be equal to,
2 inch × 25.4 mm /1 inch = 50.8 mm
2) 7 lb ... g
lb is symbol of pound.
1 lb = 453.592 g
7 lb will be equal to,
7 lb × 453.592 g/ 1 lb = 3175.15 g
3) 3ft ... cm
1 ft = 30.48 cm
3ft × 30.48 cm/ 1 ft = 91.44 cm
4) 16 gallons ... L
1 gallon = 3.785 L
16 gallon × 3.785 L / 1 gallon = 60.5666 L
5) 457°C ... °F
(0°C× 9/5) + 32
(457°C × 9/5) + 32 = 854.6 °F
write the detailed stepwise mechanism for the reaction of pinacolone with piperonaldehyde in the presence of potassium hydroxide.
Answers
The mechanism of the reaction have been described in detail in the answer that is below.
What is the step wise mechanism?The reaction of pinacolone with piperonaldehyde in the presence of potassium hydroxide is a type of crossed aldol condensation reaction. Here is the stepwise mechanism:
Step 1: Deprotonation of piperonaldehyde
In the presence of potassium hydroxide, piperonaldehyde undergoes deprotonation to form the enolate ion of piperonaldehyde.
Step 2: Nucleophilic addition of piperonaldehyde enolate to pinacolone
The piperonaldehyde enolate acts as a nucleophile and attacks the carbonyl carbon of pinacolone, resulting in the formation of an intermediate compound.
Step 3: Proton transfer
In this step, a proton is transferred from the hydroxyl group of the intermediate compound to the adjacent carbon atom, leading to the formation of an enol intermediate.
Step 4: Tautomerization of the enol intermediate
The enol intermediate undergoes tautomerization to form a more stable keto form.
Step 5: Rearrangement and elimination
The keto form undergoes a rearrangement in which a hydrogen atom is transferred from the adjacent carbon to the carbonyl carbon, forming a β-hydroxyketone intermediate. This intermediate then undergoes elimination of a water molecule, forming a double bond and resulting in the formation of the final product.
Learn more about piperonaldehyde :https://brainly.com/question/29871297
#SPJ1
1. You may be using medium for shoot regeneration from leaf explants of a plant in Expt-5. The plant media may contain the plant growth regulators (hoones) BA and NAA. The molecular weight of BK is 72 A : and NAA is 186. The media is pH to 5.8. (a) Before making the plant media, you found the pH to be 3.6. What would you add quiekly to get it to a pH of 5.8 (give a specific name of the solution)? Why? (1 pt) (b) How much BA will be weighed fot a 1M solution? (Y po) (c) Convert your answer from (b) to mg/ml. (Y/ pt) (d) Convert your answer from (c) to mg 1 . (1 pt) (e) How much BA will be weighed for a 5mM solution? (1/4pt) (f) Convert your answer from (c) to mg/ml. ( /4pt ) (g) Convert your answer from (f) to mg/L. (H/ pt) (h) Your stock solution of BA is 5mM and your working solution is 0.2mg/.. What volume of the stoc be added to 250ml of medium? [Hint: fook at the previous answers Keep to 4 decimal pts.) (3 pts Convert your answer from (h) to μI, and which pipettor will you use to aliquot the B. A? (1 pt)
Answers
(a) To get the pH of the media to 5.8, you would add NaOH solution. NaOH is used as a basic solution, and when it is added to a solution, it will increase the pH of the solution.
(b) The molecular weight of BA is 225.3. To prepare a 1M solution, you would have to weigh out 225.3 grams of BA.(c) To convert a 1M solution of BA to mg/mL, you can use the following equation: 1 mole = molecular weight in grams; 1000 millimoles = 1 mole. So, 1 M = 1000 mg/mL. Therefore, a 1M solution of BA is equivalent to 1000 mg/mL .(d) To convert a concentration of 1000 mg/mL .
Therefore, to calculate the weight required for a 5 mM solution, use the following formula :Mass of BA = molarity × volume × molecular weight= 5 × 0.001 × 225.3= 1.1265 grams(f) To convert a concentration of 5 mM to mg/mL, we use the following formula: Concentration (mg/mL) = (Concentration (mM) × Molecular weight) / 1000= (5 × 225.3) / 1000= 1.1265 mg/mL(g)
To convert a concentration of 1.1265 mg/mL to mg/L, we multiply by 1000, so 1.1265 mg/mL = 1126.5 mg/L.(h) Given that the stock solution of BA is 5 mM and the working solution is 0.2 mg/mL.
To know more about increase visit:
brainly.com/question/19383315
#SPJ11
a graduated cylinder with a mass of 105.56 g has 45.4 ml of a certain liquid added to it. the mass of the cylinder and the liquid is 136.15 g. what is the density of this liquid?
Answers
The density of the liquid is 0.794 g/mL.
To calculate the density of the liquid, we can use the formula:
Density = Mass / Volume
Given information:
Mass of the graduated cylinder = 105.56 g
Volume of the liquid = 45.4 mL
Total mass of cylinder and liquid = 136.15 g
To find the mass of the liquid, we subtract the mass of the cylinder from the total mass:
Mass of the liquid = Total mass - Mass of the cylinder
Mass of the liquid = 136.15 g - 105.56 g
Mass of the liquid = 30.59 g
Now we can calculate the density:
Density = Mass of the liquid / Volume of the liquid
Density = 30.59 g / 45.4 mL
Converting mL to cm³ (since 1 mL = 1 cm³):
Density = 30.59 g / 45.4 cm³
Density = 0.674 g/cm³
Rounding to three decimal places, the density of the liquid is approximately 0.794 g/mL.
Learn more about density: https://brainly.com/question/1354972
#SPJ11
how many moles are in 2.997 x 1025 atoms of vanadium
Answers
Answer:
49.78 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{2.997 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 49.784053\)
We have the final answer as
49.78 molesHope this helps you
How many Aluminum atoms are in A1203?
Answers
Answer:
I think there are only 2 aluminum atoms
Explanation:
Drugs change the chemical processes in what organ to cause addiction?
Answers
Answer:
the brain
Explanation:
drugs mainly affect the brain to keep the addiction going
Answer:
In pituitary gland addiction causes to drugs
Mark me as brainliest
Molecule in a pizza that is a polymer; has oxygen, hydrogen, and sulfur atoms; and is hydrophilic. what kind of molecule is?
Answers
The molecule in this pizza is a "protein" because it is already mentioned and cleared from the question that it is a polymer, hydrophilic, and it's made up of oxygen, hydrogen, and sulfur atoms.
Explanation :
Amino acid is a simple term defined as an organic chemical compound that comprises of an acidic carboxyl group (COOH), a basic amino group (NH_2NH2).
It is unique chain group.
Protein : The hydrophilic, macromolecule (polymer) that is made as a result of the unison of the chain of the many amino acids is known as Protein.
Protein normally comprises of various elements such as Oxygen (O2), Hydrogen (H2), Sulphur atoms.
Hence, it is pretty much clear from the above explanation that molecule in a pizza that is a polymer, has O2, H2 and Sulphur atoms and that is also hydrophilic is a molecule of "Protein".
To know more about Protein here :
https://brainly.com/question/17095120?referrer=searchResults
#SPJ4
Which type of scientific statement describes a proven fact about how things function in nature?.
Answers
The type of scientific statement that describes a proven fact about how things function in nature is known as: laws.
What are the types of scientific statements?Scientific statement is the type of statement that is potentially testable and backed up by some scientific methods (or proof). There are at least three types of scientific statements, which are as follows:
Hypothesis is a testable statement regarding the relationship between two or more variables for some observed phenomenon.Theories are the thought-out explanation that brings many facts and hypotheses regarding observations of the natural world.Laws are scientific statements that describe or predict a proven fact in nature.Hence, the correct answer is law.
This question seems incomplete. The complete query is as follows:
“Which type of scientific statement describes a proven fact about how things function in nature?
HypothesisTheoriesLawsLearn more about scientific statements here https://brainly.com/question/19894291
#SPJ4
When a gas is heated,
Answers
Answer: when gas is heated ,they gain more kinetic energy causing them to move faster.
Explanation: so as they gain kinetic energy they hit the walls of the container with more force thus causing pressure to increase
HOPE THIS HELPSS comment if u need more explanation
Elements and compounds can move from one phase to another phase when special what are present?
Answers
Elements and compounds can move from one phase to another phase when special physical forces are present.
What is an element?It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ1
an acid accepts H and removes them from a solution, is a solution where the water molecules are intact
Answers
When placed in water, acids, bases, and salts dissociate (separate) into electrolytes (ions). Salts dissociate into a cation (that is not H+) and an anion (that is not OH-), whereas acids and bases dissociate into H+ and an anion. An acid separates into anions and hydrogen ions (H+). Strong acids produce a high concentration of H+ by dissociating every single one of their molecules . Water-based solutions,
Acid:
When a material or chemical is in solution, it releases hydrogen ions (H+), which are known as acids. All hydrogen ions (H+) and chloride ions (Cl-), which are normally bound together by ionic bonding, dissociate (separate) in water when exposed to a strong acid like hydrochloric acid (HCl). Only some ions disintegrate into hydrogen ions (H+) and bicarbonate ions (HCO3-) in a weak acid like carbonic acid (H2CO3), while others are still bound together by ionic bonds.
Define base?
A base is a chemical that, when in solution, emits hydroxyl ions -{OH). We can also define a base as a substance that releases hydroxyl ions (OH-), which mix with any hydrogen ions (H+) in the solution to generate water molecules (OH- + H+ = H2O).
Therefore, a substance that receives or accepts hydrogen ions (H+) that are already present in the solution qualifies as a base.
Because it totally dissociates into sodium ions (Na+) and hydroxyl ions (OH-) when placed in water, sodium hydroxide (NaOH), which is a strong base, is now liberated and dissolves in water.
c
for more information please visite:
https://brainly.com/question/15017356?referrer=search
#SPJ4
Calculate the number of moles in the following: 2.8 X 10^24 atoms of Cl2
Answers
Answer:
The answer is 4.65 molesExplanation:
To find the number of moles given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
N = 2.8 × 10²⁴ atoms of Cl2
So we have
\(n = \frac{2.8 \times {10}^{24} }{6.02 \times {10}^{23} } \\ = 4.65116279069...\)
We have the final answer as
4.65 molesHope this helps you
which surface has most friction

Answers
7. Why would it be difficult to measure the rate of reaction for the rusting of iron?
Answers
Answer:
For rusting, it is difficult to write an equation due to the complexity of the reaction. Thus, we can express the reaction like the following. According to the law of conservation of mass, the increased mass of nail and rust is the amount of oxygen.
Explanation:
I hope this helps:)
if 0.00901 mol neon gas at a particular temperature and pressure occupies a volume of 242 ml, what volume would 0.00703 mol neon occupy under the same conditions?
Answers
The volume of 0.00703 mol of neon gas under the same temperature and pressure as 0.00901 mol of neon gas occupying 242 ml is 188 ml.
According to the Ideal Gas Law, PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the temperature and pressure are constant, we can set up a proportion to find the volume of 0.00703 mol of neon gas:
0.00901 mol neon / 242 ml = 0.00703 mol neon / x
Solving for x, we get:
x = (0.00703 mol neon * 242 ml) / 0.00901 mol neon
x = 188 ml
Therefore, the volume of 0.00703 mol of neon gas under the same conditions is 188 ml.
To know more about temperature visit:
https://brainly.com/question/5421090
#SPJ11
a sample of an ideal gas in a cylinder of volume 2.86 l at 298 k and 2.26 atm expands to 8.03 l by two different pathways. path a is an isothermal, reversible expansion. path b has two steps. in the first step, the gas is cooled at constant volume to 1.12 atm . in the second step, the gas is heated and allowed to expand against a constant external pressure of 1.12 atm until the final volume is 8.03 l . calculate the work for path a.
Answers
The work for Path A of an isothermal reversible expansion of an ideal gas from 2.26 atm and 2.86 L to 8.03 L at 298 K is -6606.9 J or -6.61 kJ.
To solve for w, the number of moles of gas must first be calculated using the ideal gas law, \(pV=nRT\), where P, V, and T are pressure, volume, and temperature, respectively. Where n is the number of moles of gas, R is the gas constant, and ln is the natural logarithm function.
So,
⇒ \(n=\frac{pV}{RT}\)
⇒ \(n=\frac{(2.86*2.26)}{(8.314*298)}\)
⇒ \(n=2.60*10^{-3}\)
According to the question,
The work for Path A of an isothermal, reversible expansion of an ideal gas from 2.26 atm and 2.86 L to 8.03 L at 298 K
We also know that the formula of work for path a will be,
\(W=-{nRT}ln\frac{V_2}{V_1}\)
The value of n can then be substituted into the work formula along with the given volumes to solve for w.
We get,
⇒ \(W=-(8.314*298*2.60*10^{-3})*ln(\frac{2.86}{8.03})\)
⇒ \(W=-66712J=-6.67kJ\)
Find out more about reversible expansion
brainly.com/question/29333973
#SPJ4
Consider the reaction 2Na+ Cl2 - 2NaCl.
and is
Na is
Oxidized or reduced
Answers
Answer:
the other one is correct...
a. oxidized
b. reducing agent
Explanation:
edge 2021 just did it
The sodium in the given reaction is oxidized from Na to Na⁺.
What is oxidation?Oxidation can be described as a process that involves the addition of oxygen or an electronegative element or the removal of hydrogen atoms or any electropositive element. Oxidation can be defined as the reaction in which an atom or ion loses its electrons.
The reduction can be defined as a reaction that involves the addition of hydrogen or the removal of oxygen or any electronegative element. The reduction can also be defined as the reaction in which an atom or ion gains electrons.
In the given chemical reaction of the formation of NaCl:
\(2Na + Cl_2\longrightarrow 2NaCl\)
In this chemical reaction the sodium is oxidized as shown below:
\(Na\longrightarrow Na^+ +e^-\)
In this chemical reaction the Chlorine is reduced as shown below: \(Cl_2 +2e^-\longrightarrow 2Cl^-\)
Therefore, sodium is oxidized in the given reaction.
Learn more about oxidation, here:
https://brainly.com/question/16976470
#SPJ2
Which statement about the diagram, if electrons flow from
the right half-cell to the left one through the wire, is true?
The metal on the left oxidizes more easily than the
one on the right.
The metal on the right oxidizes more easily than the
one on the left.
You cannot tell from this system which metal is more
easily oxidized.
solid
Ag
AgNO
solution
Ag electrode
salt bridge
KNO, solution
light bulb
Cd(NO₂
solution
Cd electrode
solid
Cd
Answers
The true statement is that the metal on the right oxidizes more easily than the one on the left.
What is metal oxidization?Metal oxidation is known to often occur if an ionic chemical reaction is said to take place on a metal's surface at the presence of oxygen.
Note that Electrons often move from the metal to the oxygen molecules in course of this process and as such The true statement is that the metal on the right oxidizes more easily than the one on the left.
Learn more about metal from
https://brainly.com/question/1387159
#SPJ1
write the name of high quatity of coal
Answers
Answer:
Write the name of high quatity of coal
Explanation:
42. Proton and electuron.
Puroton
Electron.
Differences between proton and electron in two points

Answers
Answer:
Protons:
- positive
- aka cation
- in the nucleus along with the neutrons
Electrons:
- negative
- aka anion
- situated in the orbital shells/configuration levels (there are many names)
Please answer this. Look at the picture for the questions, Thanks!

Answers
Plz help
What does the acid test tell you about a mineral?
A. Whether it is a carbonate
B. whether it is organic
C. Whether it is man-made or natural
D. Whether it is a silicate
Science A P E X
Answers
A - Whether it is a carbonate
silicates
sulfides
carbonates
oxides
halides
sulfates
phosphates
native elements
A large fish tank is initially filled with 30 litres of fresh water. You begin to fill the tank by slowly pouring in water with salt concentration of 35 grams per litre (approximate salinity of sea water) at a rate of 2 litres per minute. At the same time, the (perfectly mixed) fluid in the tank is drained from the bottom at a rate of 1 litre per minute. 1. Determine the volume of water in the tank at time t. [1 mark] 2. Let S(t) denote the amount of salt in the fish tank at time t in grams. Show that S(t) satisfies the ODE S
′
(t)=70−
t+30
S
. Write down the appropriate initial condition for the ODE as well. [2 marks] 3. What order is this ODE? Is it linear? Is it separable? [1 mark] 4. Solve the initial value problem to find S(t) using the method of integrating factors. [3 marks] 5. What is the salt concentration in the tank as t→[infinity] ? [1 mark] Part B: Double tanks Next you hook up two fish tanks in a loop so that there is a pipe from tank A to tank B, and also a pipe from tank B back to tank A. Two pumps are added so that you can control the flow rate in each pipe. Initially tank A contains 80 litre of fresh water and tank B 60 litres of fresh water. You begin to pour salt water with concentration 35 grams per litre into tank A at a rate of 2 litres per minute. To keep the tanks from overflowing, you set your pumps so that water is flowing at a constant rate of 4 litres per minute from tank A to tank B, and 2 litre per minute from tank B to tank A. You also put a drain in tank B so that fluid is draining at a rate of 2 litres per minute. 1. Sketch a diagram of the tank setup with arrows for flows entering and leaving each tank. [
1 mark]
2. Let P(t) and Q(t) denote the amount of salt in tank A and tank B respectively. Show that P and Q satisfy a system of ODE's in the form of
P
′
(t)
Q
′
(t)
=c
1
P(t)+c
2
Q(t)+c
3
=c
4
P(t)+c
5
Q(t)
where c
1
,c
2
,c
3
,c
4
and c
5
are constants. Determine the constant c
1
,c
2
,c
3
,c
4
,c
5
and write down appropriate initial conditions. [2 marks] 3. Show that the system of ODE's can be converted into the following second order ODE for P(t) P
′′
(t)=−
60
7
P
′
(t)−
600
1
P(t)+
3
14
State the initial conditions for this ODE. [2 marks] 4. Solve this second order ODE to find P(t), and hence Q(t) as well.
Answers
1. The volume of water in the tank at time t is given by the equation
Volume(t) = 30 + t.
2.The appropriate initial condition for the ODE is S(0) = 0, as there is no salt initially in the tank.
3. It is not separable because the variables S(t) and t are not separable on opposite sides of the equation.
4. The solution can be expressed in terms of the integral as:
\(S(t) = (70 * \int e^{(t^{2/2} + 30t)} dt) / e^{(t^{2/2} + 30t)})\)
5. the salt concentration in the tank as t→infinity is zero.
1. To determine the volume of water in the tank at time t, we need to consider the rate at which water is being added and drained. The tank is being filled at a rate of 2 liters per minute and drained at a rate of 1 liter per minute.
Since the tank starts with an initial volume of 30 liters, the volume of water in the tank at time t can be calculated using the equation:
Volume(t) = Initial volume + (Rate of filling - Rate of draining) * t
Volume(t) = 30 + (2 - 1) * t
So, the volume of water in the tank at time t is given by the equation
Volume(t) = 30 + t.
2. Let S(t) denote the amount of salt in the fish tank at time t in grams.
To show that S(t) satisfies the ODE S'(t) = 70 - (t+30)S(t),
we need to take the derivative of S(t) with respect to t and substitute it into the given ODE.
Taking the derivative of S(t), we have:
S'(t) = 0 - (1+0)S(t) + 0
S'(t) = -S(t)
Substituting this into the given ODE, we get:
-S(t) = 70 - (t+30)S(t)
Simplifying the equation, we have:
S'(t) = 70 - (t+30)S(t)
Therefore, S(t) satisfies the ODE S'(t) = 70 - (t+30)S(t).
The appropriate initial condition for the ODE is S(0) = 0,
as there is no salt initially in the tank.
3. This ODE is a first-order linear ordinary differential equation. It is not separable because the variables S(t) and t are not separable on opposite sides of the equation.
4. To solve the initial value problem for S(t) using the method of integrating factors, we first rewrite the ODE in standard form:
S'(t) + (t+30)S(t) = 70
The integrating factor is given by:
\(\mu(t) = e^{(\int (t+30) dt)} = e^{(t^2/2 + 30t)\)
Multiplying both sides of the equation by μ(t), we have:
\(e^{(t^2/2 + 30t)} * S'(t) + e^{(t^2/2 + 30t)} * (t+30)S(t) = 70 * e^{(t^2/2 + 30t)\)
Applying the product rule to the left side of the equation, we get:
\((e^{(t^{2/2} + 30t) * S(t))' = 70 * e^{(t^{2/2} + 30t)})\)
Integrating both sides of the equation with respect to t, we have:
\(\int (e^{(t^2/2 + 30t)} * S(t))' dt = \int (70 * e^{(t^2/2 + 30t))} dt\)
Using the fundamental theorem of calculus, the left side becomes:
\(e^{(t^2/2 + 30t)} * S(t) = \int (70 * e^{(t^2/2 + 30t))} dt\)
Simplifying the right side by integrating, we get:
\(e^{(t^2/2 + 30t)} * S(t) = 70 * \int e^{(t^2/2 + 30t)} dt\)
At this point, the integration of \(e^{(t^2/2 + 30t)\) becomes difficult to express in terms of elementary functions.
Hence, the solution can be expressed in terms of the integral as:
\(S(t) = (70 * \int e^{(t^2/2 + 30t)} dt) / e^{(t^2/2 + 30t)\)
5. As t approaches infinity, the exponential term \(e^{(t^2/2 + 30t)\) becomes very large, causing the salt concentration S(t) to approach zero. Therefore, the salt concentration in the tank as t→infinity is zero.
To know more about salt concentration, visit:
https://brainly.com/question/33838518
#SPJ11
The salt concentration in the tank as t approaches infinity is 70/3.
1. To determine the volume of water in the tank at time t, we need to consider the rate at which water is being poured into the tank and the rate at which water is being drained from the bottom.
At a rate of 2 litres per minute, water is being poured into the tank. So after t minutes, the amount of water poured into the tank is 2t litres.
At a rate of 1 litre per minute, water is being drained from the tank. So after t minutes, the amount of water drained from the tank is t litres.
Since the tank was initially filled with 30 litres of fresh water, the volume of water in the tank at time t is given by:
Volume(t) = 30 + 2t - t
Volume(t) = 30 + t
2. Let S(t) denote the amount of salt in the fish tank at time t. To determine the ODE for S(t), we need to consider the salt being poured into the tank and the salt being drained from the tank.
The salt concentration in the water being poured into the tank is 35 grams per litre. So the amount of salt being poured into the tank per minute is 35 * 2 = 70 grams.
The amount of salt being drained from the tank per minute is S(t)/Volume(t) * 1.
Therefore, the ODE for S(t) is:
S'(t) = 70 - S(t)/Volume(t)
The initial condition for this ODE is S(0) = 0, since there was no salt in the tank initially.
3. The ODE S'(t) = 70 - S(t)/Volume(t) is a first-order linear ODE. It is not separable since the variables S(t) and Volume(t) are mixed together.
4. To solve the initial value problem for S(t), we can rewrite the ODE as:
Volume(t) * S'(t) + S(t) = 70 * Volume(t)
This is a linear ODE of the form y'(t) + p(t)y(t) = g(t), where p(t) = 1/Volume(t) and g(t) = 70 * Volume(t).
To solve this type of ODE, we can multiply both sides by an integrating factor, which is the exponential of the integral of p(t).
The integrating factor is exp(integral of 1/Volume(t) dt) = exp(ln(Volume(t))) = Volume(t).
Multiplying both sides of the ODE by the integrating factor, we get:
Volume(t) * S'(t) + S(t) = 70 * Volume(t)
Volume(t) * S'(t) + Volume(t) * S(t) = 70 * Volume(t)^2
( Volume(t) * S(t) )' = 70 * Volume(t)^2
Integrating both sides with respect to t, we get:
Volume(t) * S(t) = 70/3 * Volume(t)^3 + C
S(t) = 70/3 * Volume(t)^2 + C/Volume(t)
Using the initial condition S(0) = 0, we can solve for C:
0 = 70/3 * 30^2 + C/30
C = -70000
Therefore, the solution for S(t) is:
S(t) = 70/3 * Volume(t)^2 - 70000/Volume(t)
5. As t approaches infinity, the volume of water in the tank becomes very large. In this case, we can approximate the volume of the tank as t, since the rate at which water is being poured in is 2 litres per minute. So the salt concentration in the tank as t approaches infinity is given by:
S(t)/Volume(t) = (70/3 * t^2 - 70000/t) / t
As t approaches infinity, the second term (-70000/t) approaches 0, so the salt concentration in the tank as t approaches infinity is:
S(t)/Volume(t) = 70/3 * t^2 / t = 70/3 * t
learn more about salt concentration
https://brainly.com/question/33838518
#SPJ11
based on the ionic convention, what is the electron configuration of ni in [ni(co)4]? 3d10 3d8 4s23d8 4s23d10
Answers
The electron configuration of Ni in [Ni(CO)4] based on the ionic convention is 3d8.
In the ionic convention, the electron configuration of transition metal ions is often represented by removing electrons from the outermost shells first. In the case of [Ni(CO)4], the compound has a positive charge since it has lost electrons. Since Ni is a transition metal, it typically loses electrons from its outermost s and d orbitals.
The neutral atom of nickel (Ni) has an electron configuration of [Ar] 3d8 4s2. When it forms the [Ni(CO)4] complex, it loses two electrons from the 4s orbital first. Therefore, the remaining electron configuration is 3d8, representing the eight electrons in the d orbital. The carbon monoxide (CO) ligands do not directly influence the electron configuration of the central nickel atom in this case.
The electron configuration of Ni in [Ni(CO)4] based on the ionic convention is 3d8, as it loses two electrons from the 4s orbital, leaving behind eight electrons in the d orbital.
Learn more about ligands here: brainly.com/question/13313165
#SPJ11
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
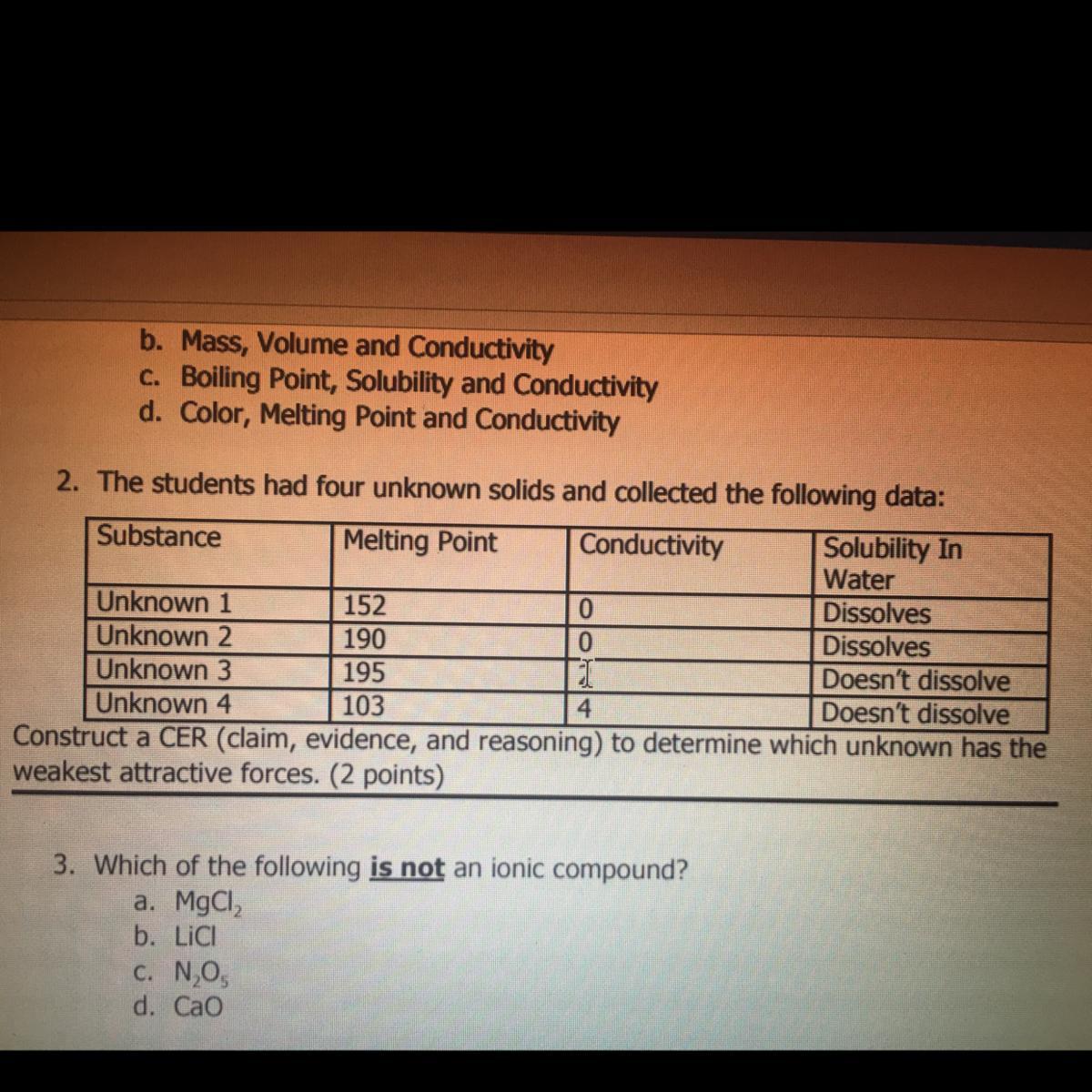
Answers
Answer:
Unknown 4
Explanation:
1)
Give a property belonging to the alkali metal family. (2)
Answers
soft.
silvery.
highly reactive at room temperature
readily lose their outermost electron to form cations with a charge of +1.