If the concentration of NaCl is 6. 07 M, when it begins to crystallize out of solution, then what is the Ksp
Answers
The Ksp of NaCl when it begins to crystallize out of a 6.07 M solution is approximately 36.84.
To calculate the Ksp of NaCl in this solution, follow these steps:
1. Identify the balanced dissociation equation: NaCl(s) ↔ Na+(aq) + Cl-(aq).
2. Since NaCl dissociates into a 1:1 ratio, the concentrations of Na+ and Cl- are equal to the initial concentration, 6.07 M.
3. Determine the Ksp expression: Ksp = [Na+][Cl-].
4. Substitute the concentrations into the expression: Ksp = (6.07)(6.07) ≈ 36.84.
In this scenario, the Ksp value represents the point at which NaCl begins to crystallize from the solution. The Ksp increases as more solute precipitates, which reflects the equilibrium between dissolved and solid NaCl.
To know more about dissociation equation click on below link:
https://brainly.com/question/30983331#
#SPJ11
Related Questions
Which pair of reactants will undergo aldol condensation to produce methylvinyl ketone?
Answers
When formaldehyde and acetone then react with each other( aldol condensation) then it will be formed methyl vinyl ketone.
In organic chemistry, an aldol condensation would be a condensation reaction in which an enol and enolate ion combines with a carbonyl chemical to produce a -hydroxy aldehyde or -hydroxy ketone, that is then dehydrated to produce a conjugated enone.
In aldol condensation, when formaldehyde and acetone then react with each other then it will be formed methyl vinyl ketone.
It can be written as
\(CH_{2} O + CH_{3} COCH_{3}\) → \(HOCH_{2} -CH_{2} -CO-CH_{3}\)
When it will be heated then it gives methyl vinyl ketones.
\(HOCH_{2} -CH_{2} -CO-CH_{3}\) → \(CH_{2} =CH-CO-CH_{3}\)
So, the pair of reactants will be formaldehyde and acetone
To know more about aldol condensation
https://brainly.com/question/9415260
#SPJ4
describe how electrical resistance readings from a colorimeter/multimeter can be used to explain the relationship between concentration and absorbance.
Answers
When using a colorimeter/multimeter, electrical resistance readings can be used to explain the relationship between concentration and absorbance as follows:
Electrical resistance readings from a colorimeter/multimeter can be used to explain the relationship between concentration and absorbance by Ohm’s law, which states that the electric current through a conductor between two points is directly proportional to the potential difference across the two points. Ohm’s law, I = V/R calculates the resistance between two points.
Here, V is voltage and R is resistance. A colorimeter/multimeter works on this principle and measures resistance, which can be used to calculate the concentration of a solution. When there is a high concentration of a substance in a solution, there will be a low resistance between two points.
Similarly, when there is a low concentration of a substance in a solution, there will be a high resistance between two points. This is because the number of particles that the current has to travel through will increase as the concentration of the solution decreases. The absorbance of a substance in a solution is proportional to the concentration of the substance. Therefore, by measuring the resistance of a solution, one can calculate the concentration of the solution. This, in turn, can be used to determine the absorbance of the substance in the solution.
Learn more about colorimeter/multimeter at https://brainly.com/question/8960388
#SPJ11
A 2.50 L balloon is filled with water at 2.27 atm. If the balloon is squeezed into a 0.80 L beaker and does NOT burst, what is the pressure of water in the balloon?
Answers
Answer:
\(P_2=7.09atm\)
Explanation:
Hello!
In this case, given the change in volume and pressure of the gas, it is possible for us to recall the Boyle's law as way to understand the inversely proportional relationship between pressure and volume:
\(P_1V_1=P_2V_2\)
Thus, when solving for the final pressure, P2, given the initial pressure and volume and the final volume, we obtain:
\(P_2=\frac{P_1V_1}{V_2}\\\\P_2=\frac{2.27atm*2.50L}{0.80L}\\\\P_2=7.09atm\)
Best regards!
Answer:
7.1 atm
Using Boyle’s law, P1 V1=P2 V2.
i need help people :0


Answers
THIS IS URGENT A 1.362 g sample of an iron ore that contained Fe3O4 was dissolved in acid and all of the iron was reduced to Fe2+. The solution was then acidified with H2SO4 and titrated with 39.42 mL of 0.0281 M KMnO4, which oxidized the iron to Fe3+. The net ionic equation for the reaction is 5Fe2+ + MnO4- + 8H+ h 5Fe3+ + Mn2+ + 4H2O (a) What was the percentage by mass of iron in the ore? (b) What was the percentage by mass of Fe3O4 in the ore?
Answers
The percentage of iron in the sample is obtained as 22.8 %.
What is the percentage by mass of iron in the ore?We can see that the reaction as it has been shown in the question is a redox reaction equation. We need to find the percent of the iron that is contained in the ore as we have in the question.
We have to obtain;
Number of moles of the permanganate = 39.42/1000 L * 0.0281 M
= 1.11 * 10^-3 moles
Then we can see from the reaction that;
If 5 moles of the iron III reacts with 1 mole of the permanganate
x moles of the iron would react with 1.11 * 10^-3 moles of the permanganate
x = 5 * 1.11 * 10^-3 /1
= 5.55 * 10^-3 moles
Mass of the iron = 5.55 * 10^-3 moles * 56 g/mol
= 0.311 g
Given that the percentage of iron = mass of iron present/ mass of iron in the sample = 0.311 g/ 1.362 g * 100/1
= 22.8 %
Learn more about percent of iron:https://brainly.com/question/26078230
#SPJ1
Plss answer the question in the picture.

Answers
Ethers undergo an acid-catalyzed cleavage reaction when treated with the Lewis acid BBr3 at room temperature. Draw the structure of the first intermediate in the reaction between BBr3 and 2-methoxybutane
Answers
The structure of the first intermediate in the reaction between BBr3 and 2-methoxybutane is as follows: CH3CH(O-BBr3)CH2CH3
The first intermediate in the acid-catalyzed cleavage reaction between BBr3 and 2-methoxybutane can be obtained through the following steps:
Identify the structure of 2-methoxybutane: It consists of a butane chain with a methoxy (OCH3) group attached to the second carbon atom.The Lewis acid BBr3 (boron tribromide) coordinates to the oxygen atom of the methoxy group, which has a lone pair of electrons. This interaction leads to the formation of a bond between the oxygen atom and the boron atom in BBr3.As a result, the first intermediate will have a BBr3 group bonded to the oxygen atom of the 2-methoxybutane.So, the structure of the first intermediate in the reaction between BBr3 and 2-methoxybutane is as follows: CH3CH(O-BBr3)CH2CH3
To know more about "Lewis acid" refer here:
https://brainly.com/question/2282910#
#SPJ11
Help with Complex Ions!
Determine the [Ag+] present in solution at equilibrium when the complexing agent ammonia is added to a 0.025 M solution of AgNO3 to result in a [NH3]eq = 0.15 M.
Solubility constant (Ksp) for Ag(NH3)2 2+ is 1.7 * 10^7.
Answers
The concentration of [Ag+] present in the solution is 6.53 × 10⁻⁸ M
From the given information, the equation for the reaction between silver ion and ammonia can be represented as:
Ag⁺ + 2NH₃ ↔ [Ag(NH₃)₂]⁺
Given that the solubility constant (Ksp) for the product formed = 1.7 × 10⁷.
The solubility constant for this reaction can be expressed as:
\(\mathbf{Ksp = \dfrac{[Ag(NH_3)_2]^+}{[Ag^+][NH_3]^2} }\)
where;
concentration of ammonia = 0.025 Mconcentration of silver nitrate = 0.15 M\(\mathbf{1.7 \times 10^7 = \dfrac{0.025}{(x)(0.15)^2}}\)
\(\mathbf{1.7 \times 10^7 = \dfrac{1.1111}{x}}\)
\(\mathbf{x = 6.54 \times 10^{-8} \ M}\)
Therefore, we can conclude that the concentration of [Ag+] = x = 6.54 × 10⁻⁸ M.
Learn more about solubility constant here:
https://brainly.com/question/17274813?referrer=searchResults
The resistance of a thermometer is 5 ohm at 25 degree Celsius and 6 2 at 50 degree Celsius. Using linear approximation, calculate the value of resistance temperature coefficient at 45 degree Celsius.
Answers
The approximate resistance value at 45 degrees Celsius is around 5.8 ohms.
To calculate the value of the resistance temperature coefficient at 45 degrees Celsius using linear approximation, we can use the formula:
R(T) = R0 + α(T - T0),
where R(T) is the resistance at temperature T, R0 is the resistance at a reference temperature T0, α is the resistance temperature coefficient, and (T - T0) is the temperature difference.
Given that the resistance at 25 degrees Celsius is 5 ohms (R0 = 5) and the resistance at 50 degrees Celsius is 6 ohms (R(T) = 6), we can calculate the value of α.
6 = 5 + α(50 - 25),
Simplifying the equation:
1 = 25α,
Therefore, α = 1/25 = 0.04 ohm/degree Celsius.
Using the linear approximation, we can approximate the value of the resistance at 45 degrees Celsius:
R(45) = 5 + 0.04(45 - 25) = 5 + 0.04(20) = 5 + 0.8 = 5.8 ohms.
Therefore, the value of the resistance at 45 degrees Celsius is approximately 5.8 ohms.
You can learn more about resistance at
https://brainly.com/question/17563681
#SPJ11
For molecules with only one central atom, how many lone pairs on the central atom guarantees molecular polarity?
Answers
Answer:
The answer is "1".
Explanation:
It is the lone pairs at, which the central atom provides the molecular and the polarity. It is only for substances with just a central atom, for which the molecules with only one central atom and only one of the solitary paired were required, that's why in this question the "1" is the correct answer.
if the density of iron is 8.76g/ml how many liters will 10.0 grams occupy please I need help
Answers
Answer:
0.876 ml
8.76/10 = 0.876
2NazN + 3MgO -> Mg3N2 + 3Na2O, what is the mole ratio of magnesium oxide to magnesium nitride?
Answers
Answer:
3:1 or 3/1
Explanation:
Look at the coefficients then set up ratio. Simplify to the base numbers and you get 3/1 or 3:1. Thus, for every 3 MgO you produce 1 Mg3N2
In the previous step, you determined
0.25 mol HCI reacts. The molar mass
of Mg is 24.31 g/mol.
What mass of Mg is required?
PLEASE HELP ASAP

Answers
Approximately 3.04 grams of magnesium would be required to react with 0.25 moles of hydrochloric acid.
To determine the mass of Mg required, we need to use the balanced chemical equation for the reaction between hydrochloric acid (HCl) and magnesium (Mg):
2HCl + Mg → MgCl2 + H2
From the balanced equation, we can see that 2 moles of HCl react with 1 mole of Mg. Therefore, if 0.25 mol of HCl reacts, we would need half of that amount, which is 0.125 mol of Mg.
To calculate the mass of Mg required, we need to multiply the number of moles of Mg by its molar mass. The molar mass of Mg is given as 24.31 g/mol. Therefore, the mass of Mg required can be calculated as follows:
Mass of Mg = Number of moles of Mg × Molar mass of Mg
Mass of Mg = 0.125 mol × 24.31 g/mol
Mass of Mg = 3.04 g
For such more questions on moles
https://brainly.com/question/19964502
#SPJ8
Q.3. (2 marks) Determine the diffusion coefficient for p type Germanium at T =300 K if you know that the carrier impurities equals to 10¹cm-³
Answers
The diffusion coefficient for p type Germanium at T =300 K if you know that the carrier impurities equals to 10¹cm-³ is 16.1 cm²/s.
In semiconductors, the diffusion coefficient is a measure of how quickly dopant atoms diffuse into the host material. In Germanium, the diffusion coefficient is found using the equation below.Using the formula below, we can determine the diffusion coefficient for p-type Germanium at T=300K.Dn= (KbTq)/µnIt is essential to note that for p-type dopant, the mobility value is different from the electron value.
The electron mobility value is µn while the hole mobility value is µp. Using the information provided in the question that the carrier impurities equal to 10¹ cm-³ and the temperature, we can use the following values to calculate the diffusion coefficient for p-type Germanium at T=300K. Dp = (KbTq)/µp (Nd) = (1.38 × 10−23 J/K × 300 K × 1.6 × 10−19 C)/( 1600 cm²/Vs) (10¹ cm-³) = 16.1 cm²/s.
Therefore, the diffusion coefficient for p-type Germanium at T=300 K with carrier impurities equals to 10¹cm-³ is 16.1 cm²/s.
Learn more about diffusion coefficient at:
https://brainly.com/question/31430680
#SPJ11
Calculate the molality of a solution containing 275.0-grams of methane, CH4, dissolved in 300.0-m L of water.
Answers
The molality of a solution containing 275.0-grams of methane, CH₄, dissolved in 300.0-m L of water is 57.3 M.
What is the molarity of the solution?The molality of a solution containing 275.0-grams of methane, CH₄, dissolved in 300.0-m L of water is calculated as follows;
The molarity of a solution is defined as the ratio of number of moles of solute to the volume of the solution in liters.
The number of moles of 275.0-grams of methane, CH₄ is;
n = 275 / 16
n = 17.19 moles
The volume of the solution in liters = 0.3 L
The molality of a solution containing 275.0-grams of methane, CH₄, dissolved in 300.0-m L of water is ;
= 17.19 moles / 0.3 L
= 57.3 M
Learn more about molarity here: https://brainly.com/question/14469428
#SPJ1
if you were given lithium and beryllium, predict which element would react more vigorously with water to produce hydrogen gas. explain your answer. (3 pts)
Answers
Lithium is the element that reacts more vigorously with water to produce hydrogen gas.
Explanation: The reactivity of metals with water is referred to as "metal-water reaction." Hydrogen gas is produced when a metal reacts with water. Lithium, which is an alkali metal, is highly reactive and will react with water at normal temperatures; hence, it is the most reactive of the alkali metals. Beryllium, on the other hand, does not react with water because its surface is protected by an oxide layer formed by air. The reaction of lithium with water produces a white solid compound known as lithium hydroxide and hydrogen gas. The reaction equation is as follows: 2Li(s) + 2H2O(l) → 2LiOH(aq) + H2(g)The reaction is highly exothermic and releases a lot of heat. As a result, the hydrogen produced may ignite, and the reaction may become explosive. This makes lithium highly reactive with water, producing hydrogen gas as well as fire. This is the reason why lithium metal is usually stored in oil to protect it from the water vapor present in the atmosphere.
Learn more about element would react more vigorously with water at: brainly.com/question/29457858
#SPJ11
A can contains a gas with a volume of 86 mL at 30oC. What is the volume in the can if it is heated to 65oC?
Answers
Answer:
New volume of gas = 95.93 ml (Approx)
Explanation:
Given:
Old volume of gas = 86 ml
Old temperature = 30°C = 30 + 273 = 303 K
New temperature = 65°C = 65 + 273 = 338 K
Find:
New volume of gas
Computation:
V1T2 = V2T1
(86)(338) = (V2)(303)
New volume of gas = 95.93 ml (Approx)
A gaseous mixture of methane , ethane and propane has partial pressures respectively of 95 kPa,105 kPa and 50 kPa. What is the mass percentage of methane in the mixture?
Answers
The mass percentage of methane in the gaseous mixture of methane, ethane, and propane is approximately 43.5%.
The mass percentage of a gas in a mixture can be calculated by dividing the mass of the gas by the total mass of the mixture and then multiplying by 100%.
To calculate the mass of each gas, the ideal gas law, PV = nRT, can be used. Here, P is the partial pressure of the gas, V is the volume of the mixture, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature.
Assuming a constant temperature and volume, the number of moles of each gas can be determined from the partial pressures of the gases:
n_methane = (95 kPa * V) / (R * T)
n_ethane = (105 kPa * V) / (R * T)
n_propane = (50 kPa * V) / (R * T)
The mass of each gas can be calculated using the molar mass of the gas:
m_methane = n_methane * M_methane
m_ethane = n_ethane * M_ethane
m_propane = n_propane * M_propane
Where M is the molar mass of the gas. The molar mass of methane is 16.04 g/mol, the molar mass of ethane is 30.07 g/mol, and the molar mass of propane is 44.10 g/mol.
The total mass of the mixture can be calculated as the sum of the masses of each gas:
total mass = m_methane + m_ethane + m_propane
Finally, the mass percentage of methane in the mixture can be calculated as:
mass percentage of methane = (m_methane / total mass) * 100%
Approximating the values, the mass percentage of methane in the gaseous mixture of methane, ethane, and propane is approximately 43.5%.
To learn more about propane, visit:
https://brainly.com/question/25008296#
#SPJ11
Which of the following conversion factors is correct to convert 0.25 moles of Na to particles of Na?
Answers
Answer:
6.02×10²³ particles / 1 mol
Explanation:
In this question the choices are missed.
For determine the particles of Na, in 0.25 moles we should know that the mole referrs to the Avogadro's number.
1 mol of anything contains 6.02×10²³ entities, that's why we should apply this conversion factor to find the final answer:
0.25 mol . 6.02×10²³ particles / 1 mol = 1.50×10²³ particles
In conclussion: 1.50×10²³ particles of Na are contained in 0.25 moles of Na
in the reaction between co and fe304 the theoretical yield in an experiment is calculated to be 47.2 g fe. when a careless chemistry student carries out the experiment, the actual yield is 42.9 g fe. calculate the percentage yield.
Answers
The percentage yield for the reaction is 99.88% .
The formula for the percentage yield is mentioned below;
Percent yield = actual yield / theoretical yield x 100% equation 1
Theoretical yield = 47.2 g
Actual yield = 42.9 g
If the theoretical yield of the reaction is 47.2 g Fe, we assume it as 100%, then
47.2 g Fe -----------------> 100 %
Using unitary method , we can also write this as
1g Fe -----------------> (100/47.2) %
42.9g Fe -----------------> (100/47.2) × 42.9 %
42.9g Fe -----------------> 99.88 %
Or using equation1 , we can simply write this as
Percent yield = (42.9/47.2)×100 = 99.88%
Thus, we can say 99.88 % is the percentage yield for the reaction.
To know more about percentage yield refer to the link:
https://brainly.com/question/11963853
#SPJ4
4.16 Unit Test: Chemical Bonding - Part 2 1. You are studying alkaline earth metals bonding with Halogens. Using your knowledge of the ionic bonding, what specific and testable scientific question could be asked about the salt formation
Answers
A testable scientific question could be asked about the salt formation is; "Is the salt soluble in water?
A scientific question is a question that can be answered by experiments. In other words, a scientific question must be empirical. Its answer can only be obtained by carrying out experiments.
In the case of bonding in alkaline earth metals, the kind of bond formed is ionic. Ionic compounds are soluble in water. Hence, a testable scientific question could be asked about the salt formation is; "Is the salt soluble in water?
This can be answered by dissolving several salts of alkali earth metals in water.
Learn more: https://brainly.com/question/967776
what does "8 is great" mean as it pertains to valence electrons?
Answers
Answer:
stability and completely filled orbitals
Explanation:
As it pertains to valence electrons, "8 is great" means the valence electrons present in the orbital confers stability on the atom as the shell becomes completely filled.
For an atom to be stable, it must have completely filled outer orbitals. The number of electrons required is usually 8. This is the octet number which confers special stability on an atomic configuration. Elements having this number of electrons in their outer shell are unreactive.Please answer all and I will give you 50 points, you need to pick one for each

Answers
Answer:
1. 3
2. 2
3. 4
4. 1
Explanation:
in a neutralization reaction involving an acid and a base, what do you always find as one of the products?
Answers
In a neutralization reaction involving an acid and a base, one of the products is always water.
Neutralization reactions occur when an acid reacts with a base, resulting in the formation of a salt and water. The acid donates a proton (H+) to the base, which accepts the proton. The combination of the positively charged hydrogen ion (H+) from the acid and the negatively charged hydroxide ion (OH-) from the base forms water (H2O). This water molecule is a byproduct of the neutralization reaction. The salt formed in the reaction depends on the specific acid and base involved. For example, if hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the salt formed is sodium chloride (NaCl).
To learn more about neutralization reaction click here : brainly.com/question/27745033
#SPJ11
Implement a function two_list that takes in two lists and returns a linked list. The first list contains the values that we want to put in the linked list, and the second list contains the number of each corresponding value. Assume both lists are the same size and have a length of 1 or greater. Assume all elements in the second list are greater than 0.
Answers
In a system, there are 2 singly linked lists. Another of the linked lists' end nodes was accidentally linked to a second list, creating an inverted E s list. Create a program to find the intersection of two linked lists.
Describe linked list?The head of both the linked list is the first node. The value of said neck points to NULL if somehow the link list is empty. In a list, each node has at least two components:
The head of a list refers to the point at which a linked list can be accessed. Head is not an distinct node, it should be highlighted.
Definition of comparable value?Items that show up in the same location in two related circumstances are said to be corresponding objects. Angles are a common example, as demonstrated here. Because they are located in the same spot in the two related shapes, angle A here on left corresponds to angle K on the right. We claim K is the equivalent of A.
To know more about comparable value visit:
https://brainly.com/question/24405173
#SPJ4
please tell me if i did it right. did i put the right electric charge
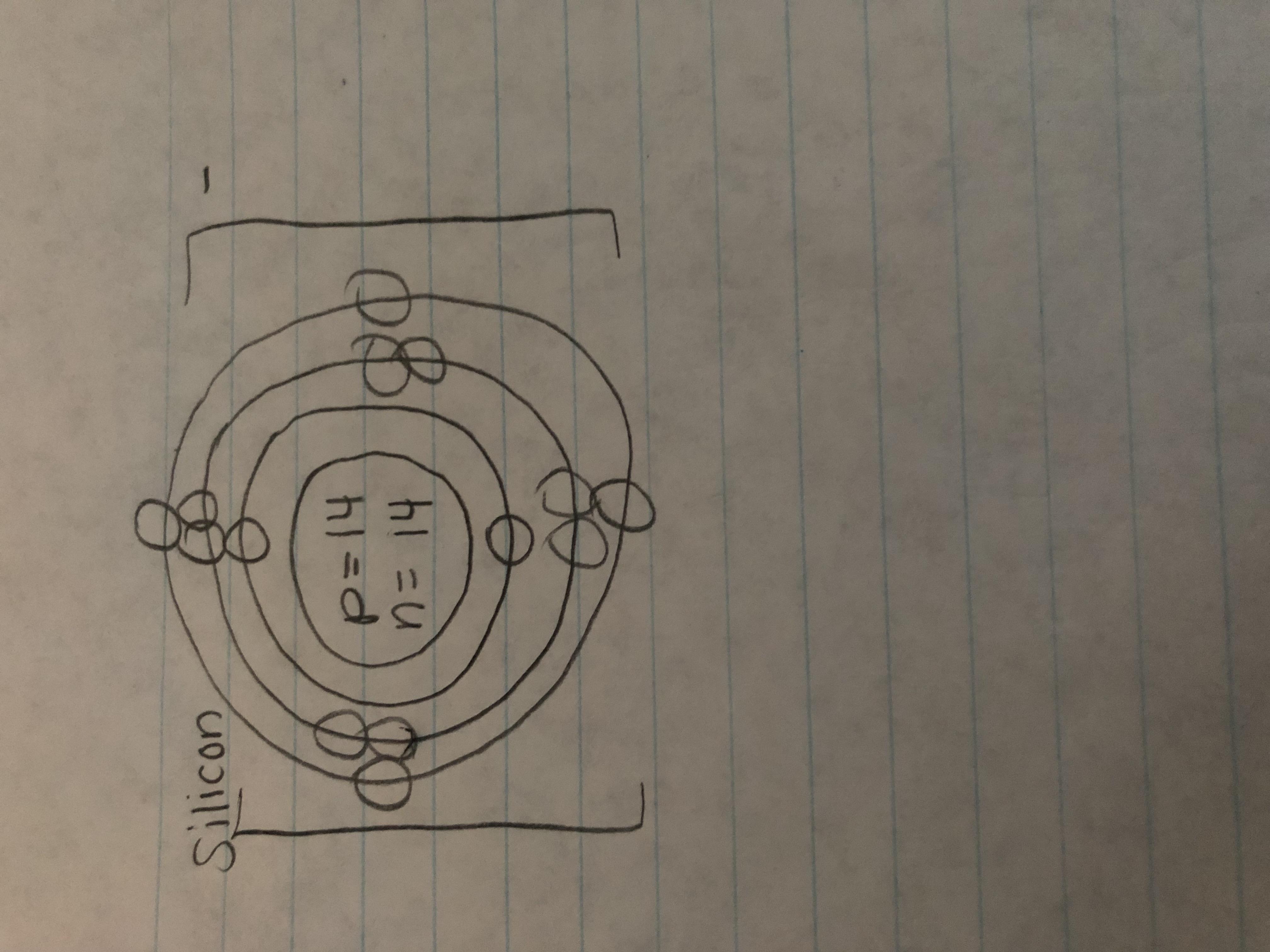

Answers
Answer:
its good but your answer
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
Does a reaction occur when aqueous solutions of magnesium nitrate and iron(ii) sulfate are combined?
Answers
Yes, a reaction occurs.
When Magnesium nitrate Mg(NO3)2 and iron (II) sulphate (FeSO4) are combined, double displacement reaction takes place.
Double displacement reaction - A reaction in which two compounds exchange their cations or anions (ions) to form two new compounds.AB + CD → AD + CB
Ionic equation according to the question-
FeSO4 + Mg(NO3)2 → Fe(NO3)2 + MgSO4
Here, Iron (II) sulphate reacts with Magnesium nitrate to form Iron nitrate and magnesium sulphate by undergoing double displacement reaction.
To learn more about double displacement reaction refer - https://brainly.com/question/10210271
#SPJ4
Yes, there is a reaction when iron (II) sulphate (FeSO₄) and magnesium nitrate Mg(NO₃)₂ are mixed.
Double displacement reaction occurs when iron (II) sulphate (FeSO₄) and magnesium nitrate Mg(NO₃)₂ are mixed.
Displacement reaction- When a less reactive element is pushed out of a compound that also contains a more reactive ingredient, the result is a displacement reaction. The less reactive element is now pure and left alone following a displacement reaction.
AB + C → A + CB
whereas,
Double displacement reaction- In a double displacement reaction, two compounds swap cations or anions (ions) to create two new molecules.
AB + CD → AD + CB
FeSO₄ + Mg(NO₃)₂ Fe(NO₃)₂ + MgSO₄ is the ionic equation in response to the query.
Here, a double displacement reaction occurs between magnesium nitrate and iron (II) sulphate to produce iron nitrate and magnesium sulphate.
To learn more about double displacement reaction refer- https://brainly.com/question/10210271
#SPJ4
wat would happen if there was no light outside???????
Answers
Answer: Without the Sun's rays, all photosynthesis on Earth would stop. All plants would die and, eventually, all animals that rely on plants for food — including humans — would die, too..
a mixture of he , ar , and xe has a total pressure of 2.50 atm . the partial pressure of he is 0.250 atm , and the partial pressure of ar is 0.400 atm . what is the partial pressure of xe ? express your answer to three significant figures and include the appropriate units.
Answers
The partial pressure of Xe will be 1.85 atm.
How to find the partial pressure?
The total pressure is equal to the sum of the partial pressures. The total pressure is equal to the sum of the partial pressures.
Subtract the sum of the known partial pressures of the other components from the overall pressure to determine the partial pressure of one component in a mixture.
Given values: P_{He} = 0.250 atm, P_{Ar} = 0.400 atm, P_{total} = 2.50 atm
To Find: P_{Xe} = ?
Solution: Calculate the total pressure, then deduct the sum from the partial pressures of helium and argon.
P_{He} + P_{Ar} = 0.250 atm + 0.400 atm = 0.650 atm
P_{Xe} = P_{total} - (P_{He} + P_{Ar}) = 2.50 atm - 0.650 atm
P_{Xe} = 1.85 atm
Learn more about the topic partial pressure: https://brainly.com/question/13199169
#SPJ4