If the pressure of a 7. 2 liter sample of gas changes from 735 torr to 800 torr and the temperature remains
constant, what is the new volume of the gas? (6. 62 L)
Answers
Answer:
you equate the question 800×7.2 divide the answer by 735.And you'll get 7.84litre then covert to 0.0m³ if the question says so to get 0.00784
Related Questions
Which term describes this reaction? upper c upper h subscript 3 upper b r (a q) plus upper o upper h superscript minus (a q) right arrow upper c upper h subscript 3 upper o upper h (a q) plus upper b r superscript minus (a q). addition condensation elimination substitution
Answers
The reaction is a substitution reaction since bromide ion was replaced by hydroxide ion.
What is a chemical reaction?A chemical reaction is a change which results in a the permanent rearrangements of the atoms and constituents of a substance such that new substances are formed.
The given reaction is a as follows:
\(CH_3Br (aq) + OH^{-} (aq) \rightarrow CH_3OH (aq) + Br^{-} (aq) \\ \)
Bromide ion was replaced by hydroxide ion.
Therefore, the reaction is a substitution reaction.
Learn more about chemical reactions at: https://brainly.com/question/16416932
#SPJ4
Answer: D-substitution
Other person is right its
Explanation:
True or false: Sodium balance and water balance are closely related; chloride levels are closely associated with changes in sodium levels. Group of answer choices True False
Answers
The statement "Sodium balance and water balance are closely related; chloride levels are closely associated with changes in sodium levels" is true because sodium balance and water balance in the body are closely interconnected.
Sodium is an essential electrolyte that plays a crucial role in maintaining fluid balance. When there is a change in sodium levels, it can lead to alterations in water balance within the body. This is because sodium helps regulate the movement of water across cell membranes, affecting the overall distribution of fluids.
Chloride, another electrolyte, is closely associated with sodium levels. Chloride ions often accompany sodium ions in various physiological processes. Changes in sodium levels can impact chloride levels, and vice versa. Therefore, chloride levels are closely linked to alterations in sodium levels, further emphasizing the relationship between sodium balance and water balance in the body.
You can learn more about Sodium at
https://brainly.com/question/28580895
#SPJ11
What would be the mass of 3 moles of water molecules?
a
54 grams
b 40 grams
C
30 grams
d 20 grams
Answers
Answer:
\(\boxed {\boxed {\sf A. \ 54 \ grams }}\)
Explanation:
To convert from moles to grams, we must use the molar mass.
Recall that water's molecular formula is H₂O. It contains hydrogen and oxygen. Look up the two elements masses on the Periodic Table.
Hydrogen (H): 1.008 g/mol Oxygen (O): 15.999 g/molNow, use these masses to find water's mass. The subscript of 2 tells us there are 2 atoms of hydrogen, so we multiply hydrogen's mass by 2 and add oxygen's.
H₂O= 2(1.008 g/mol) + 15.999 g/mol = 18.015 g/molUse the molar mass as a ratio.
\(\frac{18.015 \ g \ H_2 O}{ 1 \ mol \ H_2 O}\)
Multiply by the given number of moles.
\(3 \ mol \ H_2O*\frac{18.015 \ g \ H_2 O}{ 1 \ mol \ H_2 O}\)
The moles of water will cancel.
\(3 *\frac{18.015 \ g \ H_2 O}{ 1 }\)
\(3 *{18.015 \ g \ H_2 O}\)
\(54.045 \ g \ H_2O\)
Round to the nearest whole number. The 0 in the tenth place tells us to leave the number as is.
\(54 \ g \ H_2O\)
There are about 54 grams of water in 3 moles.
Answer:
54 grams
Explanation:
First step is to find what one mole of water is in grams.
Water is H2O so that means we have two moles of Hydrogen.
H - 2 grams (on periodic table)
We have one mole of Oxygen.
O - 16 grams (on periodic table)
Add 2 and 16 to get 18 total grams in one molecule of H2O
Now multiply 3 times 18 to know how many grams in 3 moles of H2O
You end with
3x18=54 grams
How would the particles of a solid, liquid, and gas ALL at room temperature compare? *
A.solid particles have lower average kinetic energy
B.the gas particles are larger than both solid and liquid
C.the pas particles are moving the slowest
D.the spacing of the particles is different, but all have the same average kinetic energy
Plz help
Answers
Answer:
A
Explanation:
Solid particles only vibrate, so they have very little kinetic energy.
For B, the size of the particles stay the same, just phase/state changes, so not B.
For C, gas particles move the fastest compared to liquid and solid given the same environment.
For D, they don't have the same kinetic energy because solid particles barely move while gas particles keep moving.
Suppose the interaction between two atoms by the Lennard-Jones potential: ULJ = B/r^12 - A / r^6 where the values of A and B are known to be A = 10^-77 J x m^6 and B = 10^-134 J x m^12.
What does the Lennard-Jones potential predict for the separation r=r eq
hen the energy is at the minimum (equilibrium) value, U min. What is the u min fot this interaction at T=298 K ? What is the ratio of U min to the purely attractive van der Waals component of the interaction potential at r eq.
What is the ratio of r eq to r 0 defined by u(r 0 )=0. 4. What is the ratio of r s to r 0 , where r s is the separation where the magnitude of the (attractive adhesion) force is maximum, F max . What is the value for F max between the two atoms?
Answers
a) The Lennard-Jones potential predicts the separation r_eq at the minimum energy U_min.
b) The U_min for this interaction at T=298 K is the value obtained from the Lennard-Jones potential equation when r=r_eq.
c) The ratio of U_min to the purely attractive van der Waals component of the interaction potential at r_eq can be calculated by comparing the attractive part (-A/r^6) to the total potential energy U_min.
d) The ratio of r_eq to r_0, where u(r_0)=0.4, can be determined by finding the value of r_eq where the potential energy is equal to 0.4 times the total potential energy at r=r_0.
e) The ratio of r_s to r_0, where r_s is the separation where the magnitude of the attractive adhesion force is maximum, can be determined by finding the value of r where the derivative of the potential energy with respect to r is equal to zero.
f) The value of F_max between the two atoms can be obtained by taking the negative derivative of the potential energy equation with respect to r and evaluating it at r=r_s.
a) The Lennard-Jones potential provides information about the relationship between energy and separation between two interacting atoms.
At the minimum energy (U_min), the potential predicts the separation r_eq, which corresponds to the equilibrium distance between the atoms. This is the distance at which the energy of the system is at its lowest point.
b) To determine the value of U_min at a given temperature (T=298 K), you can substitute the equilibrium separation r_eq into the Lennard-Jones potential equation and calculate the resulting energy value.
This will give you the U_min for the interaction.
c) The Lennard-Jones potential consists of two components: an attractive component (-A/r^6) and a repulsive component (B/r^12).
The ratio of U_min to the purely attractive van der Waals component of the interaction potential at r_eq can be calculated by comparing the magnitude of the attractive component to the total potential energy at the equilibrium separation.
This ratio provides insights into the relative contribution of the attractive force to the overall potential energy at equilibrium.
d) The ratio of r_eq to r_0 can be determined by finding the value of r_eq where the potential energy is equal to 0.4 times the total potential energy at r=r_0.
In other words, you need to solve the Lennard-Jones potential equation for r_eq when the potential energy is equal to 0.4 times the potential energy at r=r_0.
e) The ratio of r_s to r_0 is obtained by finding the value of r where the magnitude of the attractive adhesion force is maximum.
This can be determined by finding the separation r where the derivative of the potential energy equation with respect to r is equal to zero.
The value of r_s represents the separation at which the attractive force between the atoms is strongest.
f) The value of F_max between the two atoms can be obtained by taking the negative derivative of the Lennard-Jones potential energy equation with respect to r and evaluating it at r=r_s.
This will give you the magnitude of the maximum attractive adhesion force between the atoms.
To know more about "Lennard-Jones potential" refer here:
https://brainly.com/question/32318368#
#SPJ11
If a metal becomes an ion, what happens?
A. It gains protons and becomes positively charged
B. It loses protons and becomes negatively charged
C. It gains electrons and becomes negatively charged
D. It loses electrons and becomes positively charged
Answers
Which elements in the periodic table are least likely to combine with other elements?
a. halogens
b. Nobel gases
c. alkaline earth metals
d. transition metals
Answers
Answer:
nobel gases
Explanation:
nobel gases elements in the periodic table are least likely to combine with other elements
how are ionic bonds formed between k and br
Answers
Ionic bonds are formed between K and Br due to losing and gaining of electrons.
Ionic bond is the bond formed between two elements due to the gaining and losing of electrons by the one member and acceptance by the other member.
Usually the metal atom loses electron and the non-metal gains electron.
Ionic bond is formed between Potassium (K) and Bromine (Br). Potassium loses electron to form K⁺ and Bromine gains electron to form Br⁻ .
Potassium Bromide (KBr) is formed due to formation of K⁺ and Br⁻ after the addition of electron is Br and losing of electron by K atom.
Potassium Bromide can be Electrolysed into the constituent ions on addition of water due to its nature as a strong electrolyte.
Learn more about Ionic Bonds here, https://brainly.com/question/11527546
#SPJ9
determine the heat required to raise the temperature of 5.3 mole of water from 75.0 oc to 125 oc at atmospheric pressure. note that water transitions from the liquid to the vapor state. express your answers in joules. for your reference, and for uniformity, you may find the following pertinent: ch2o(l)
Answers
It requires 19975.7 J of heat capacity to raise 5.3 moles of water from 75.0 °C to 125 °C at atmospheric pressure.
How should I define "thermal capacity"?
Heat capacity is the quantity of heat energy required to raise a particular quantity of matter's temperature by one degree Celsius. The size or quantity of a particular substance has an impact on a general property known as heat capacity. The units used to assess heat capacity are joules per degree Celsius or joules per Kelvin.
What kind of heat capacity is that?
Thus, a substance's ability to conduct heat is an innate property. For instance, water has an incredibly high heat capacity of 4184 J per kilogram. This indicates that 4184
Briefing:
H = ncθ
m = Number of moles of water
c = Molar heat capacity of water
θ = Temperature rise
Substituting values;
H = 5.3 mole × 75.38 J/mol °C × ( 125oC - 75.0oC)
H = 19975.7 J
To know more about heat capacity visit:
https://brainly.com/question/28302909
#SPJ4
Which option is a compound?
Answers
Examples of compounds include table salt or sodium chloride , sucrose (a molecule, nitrogen gas, a sample of copper, and water.
why is methane the simplest alkane?
Answers
A neutral cloth and a neutral rod are rubbed together. Electrons are transferred from the rod to the cloth. What will be the final charge on the cloth?
Answers
Answer: The net charge on the cloth with be negative.
Explanation:
Because electrons are being transferred from the neutral rod to the neutral rod to the neutral cloth, the cloth will become negative due to the excess electrons. (The rod will also become positively charged, but that’s beyond the scope of the question.)
When a neutral cloth and a neutral rod are rubbed together then the final charge on the cloth is negative.
What is transfer of charge from material ?Whenever electrons are transferred between objects, neutral matter becomes charged. For example, when atoms lose or gain electrons they become charged particles called ions. Three ways electrons can be transferred in a material that are conduction, friction, and polarization.
How does the charge transfer from rod to the cloth ?Rubbing a neutral rod with a neutral cloth also transfer charge from both the material. We know that a metal rod is a conductor of electricity whereas the cloth is an insulator that does not conduct electricity. When both the rod and cloth is rubbed, electrons are transferred due to friction on the material. Electrons are always negatively charged. As the rod and cloth is rubbed, the electrons flow from the rod to the cloth thereby making the rod as positively charged and the cloth as negatively charged.
Thus, when a neutral cloth and a neutral rod are rubbed together then the final charge on the cloth is negative.
To learn more about transfer of charge, refer -
https://brainly.com/question/20342415
#SPJ2
Explain biomass combustion and energy recovery using grate
furnace or fluidized bed systems
Answers
Biomass combustion is referred to as a process in which organic materials are burnt and their remains are used to produce energy.
The process of combustion is very simple it refers to the burning of biomass which include wood, farm waste, and crops which are further used to produce or generate energy in the form of electricity and also heat, it can be termed as renewable energy that utilized the energy of biomass to produce another form of energy.
The Grate furnace method is one of the common methods used for biomass combustion and comprises several steps for the recovery of energy.
The first step consists of drying up the biomass by removing all the moisture using heat. The next step includes the production of flames and heat by combusting hydrogen present in it. After that, the remaining solid waste will undergo combustion in the presence of oxygen.
The last step includes the disposal of ash which gets accumulated due to incombustible materials like sand.
Learn more about combustion
https://brainly.com/question/23992512
The sulfur hexafluoride molecule consists of one sulfur atom and six fluorine atoms. The atomic masses of sulfur and fluorine are 32.0 u and 19.0 u respectively. One mole of this very heavy gas has what mass?
Answers
The sulfur hexafluoride molecule consists of one sulfur atom and six fluorine atoms. The atomic masses of sulfur and fluorine are 32.0 u and 19.0 u, respectively. One mole of this very heavy gas has a mass of 146.0 grams.
How to determine the mass of a molecule?To find the mass of one mole of sulfur hexafluoride (SF6) molecule, we need to calculate the molecular mass of the compound using the atomic masses of sulfur and fluorine.
The sulfur hexafluoride molecule consists of one sulfur atom and six fluorine atoms. The atomic masses of sulfur and fluorine are 32.0 u and 19.0 u, respectively.
Step 1: Calculate the mass of the sulfur atom.
Mass of sulfur = 1 x atomic mass of sulfur = 1 x 32.0 u = 32.0 u
Step 2: Calculate the mass of the six fluorine atoms.
Mass of fluorine = 6 x atomic mass of fluorine = 6 x 19.0 u = 114.0 u
Step 3: Calculate the molecular mass of sulfur hexafluoride.
Molecular mass of SF6 = mass of sulfur + mass of fluorine = 32.0 u + 114.0 u = 146.0 u
Step 4: Convert the molecular mass to molar mass.
One mole of sulfur hexafluoride has a mass of 146.0 grams.
To know more about Molar Mass:
https://brainly.com/question/12971094
#SPJ11
Please help! Will mark brainliest!
I’m doing exams please hurry!
The graph below plots the temperature and luminosity of stars on the main
sequence.
Which of these stars had an initial mass greater than the initial mass of the sun?
Star 1 and Star 2
Star 1 and Star 4
Star 2 and Star 3
Star 3 and Star 4

Answers
Answer:
Star 1 and two. Good on exams I'm doing mine two
Answer:star 1 and 2
Explanation:
How are chemical equations balanced?
Answers
I need help with this!!! And please explain
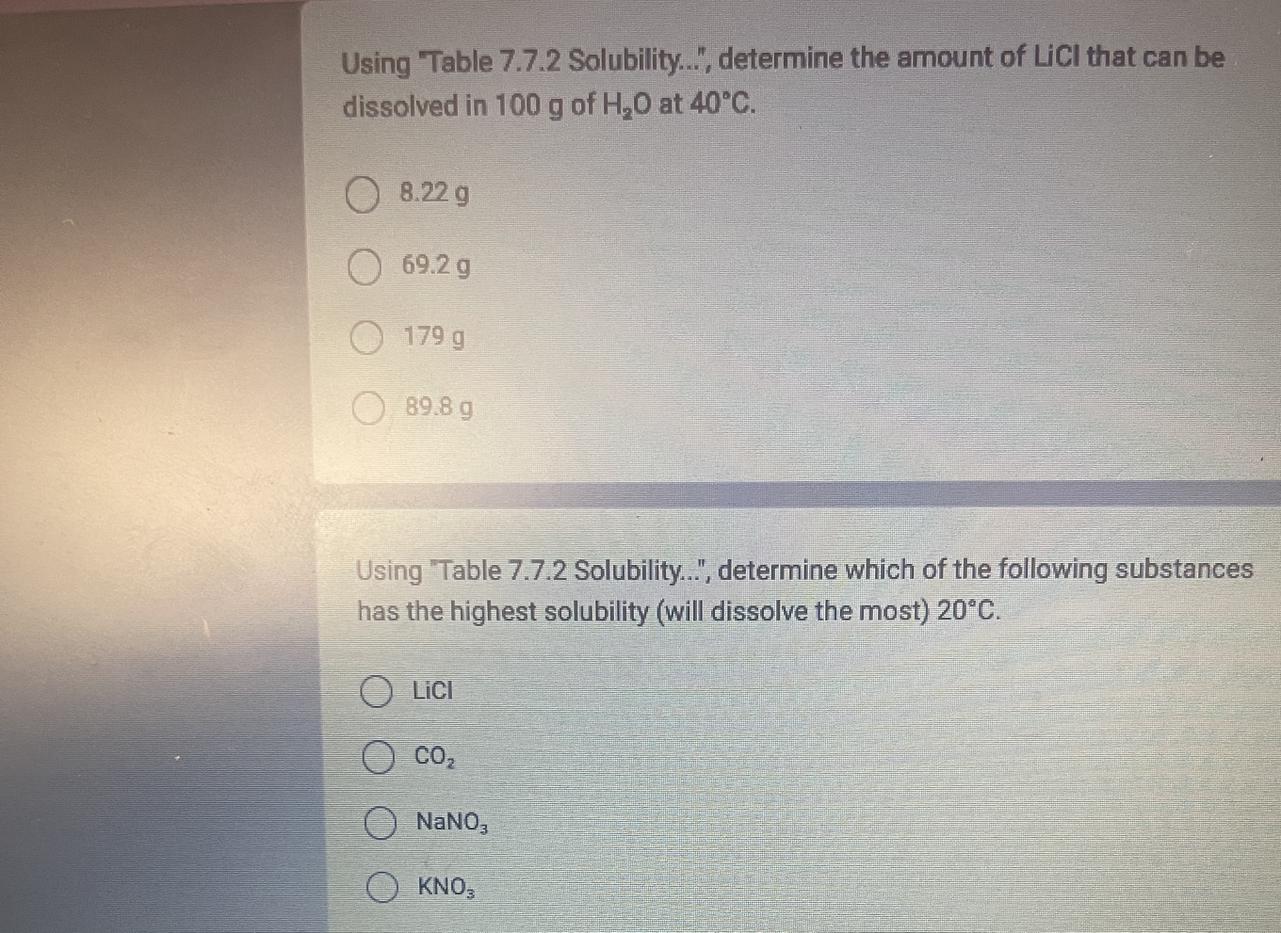
Answers
The solubility of LiCl is 89.8 g
The highest solubility is NaNO3
How do you know solubility?The solubility of a substance is a measure of how much of that substance can dissolve in a given solvent at a given temperature and pressure. Solubility can be determined experimentally by adding a known amount of the substance to a fixed amount of solvent and measuring the concentration of the solute in the resulting solution.
The solubility of a substance is usually expressed in units of grams of solute per liter of solvent (g/L) or in units of moles of solute per liter of solvent (mol/L).
Learn more about solubility:https://brainly.com/question/29661360
#SPJ1
Which of these is a covalent compound?
A. LiCl
B. MgO
C. AlCl3
D. CO
Answers
Answer:
C. AlCl3 is a covalent compound.
Explanation:
It is formed by mutual sharing of electrons between two atoms each contributing equal number of electrons to the electron pair.
Anti-glycolytic training avoids metabolic fatigue to ensure the primary source of energy is:____.
Answers
Anti-glycolytic training avoids metabolic fatigue to ensure the primary source of energy is: fat. Anti-glycolytic training typically involves longer, low-intensity exercises that rely on aerobic metabolism.
The primary source of energy in anti-glycolytic training is fat. This type of training aims to minimize the production of lactic acid, which is a byproduct of the breakdown of glucose. By doing so, it helps to improve fat oxidation and enhance endurance performance. Anti-glycolytic training typically involves longer, low-intensity exercises that rely on aerobic metabolism.
This approach allows the body to use fat as the primary fuel source, sparing glycogen and reducing metabolic fatigue. Overall, the goal of anti-glycolytic training is to enhance the body's ability to utilize fat for energy and improve endurance capacity.
To know more about aerobic metabolism, refer
https://brainly.com/question/31454892
#SPJ11
A 0.65 l(v1) balloon is filled with helium to a pressure of 101.3 kpa (p1) if the pressure of the gas increases to 2 atm (p2),what is the new volume of the balloon (v2)
Answers
Dalton's law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases
How do I calculate partial pressure?Partial pressures can be calculated in one of two ways: 1) To determine the individual pressure of each gas in a mixture, use PV = nRT. 2) Determine the proportion of pressure from the total pressure that may be assigned to each individual gas by using the mole fraction of each gas.
Pressure overall is 98.8 kPa. Each gas's partial pressure relates to how many moles of that gas there are in the combination. Therefore, the partial pressure of each gas is equal to (0.500/0.750) x 98.8 = 65.9 kPa for H2 (increased)
Because pressure and force are connected, you can determine one using the physics formula pressure = force/area if you know the other.
To be learn more about force refer to:
https://brainly.com/question/13191643?referrer=searchResults
#SPJ4
What was the carbon cycle on the prairie like?
Answers
Answer:
Explanation:
Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. ... Carbon atoms are then released as carbon dioxide when organisms respire. The formation of fossil fuels and sedimentary rocks contribute to the carbon cycle for very long periods.
A 20-milliliter sample of a gas is at 546 K and has a pressure of 6.0 atmospheres. If the temperature is changed to 273 K and the pressure to 2.0 atmospheres, the new volume of the gas
Answers
Answer: 30mL
Explanation:
A 20-milliliter sample of a gas is at 546 K and has a pressure of 6.0 atmospheres. If the temperature is changed to 273 K and the pressure to 2.0 atmospheres, the new volume of the gas is 30mL.
What is volume?Because gases occupy their containers, overall volume is identical to the container's internal volume. Liquids are typically measured using containers in which the volume or internal form of the container is indicated.
The volume of typical solid forms may be calculated using formulae. Another way to calculate the capacity of a solid would be to calculate how so much liquid something displaces.
P₁×V₁/T₁=P₂×V₂/T₂
substituting all the given values in the above equation, we get
6.0×20/ 546=2.0×V₂/ 273
V₂= 30mL
Therefore, 30mL is the final volume.
To learn more about volume, here:
https://brainly.com/question/13807002
#SPJ6
Which of these statements is supported by evidence in both articles? A The Triangle Fire was a tragedy that could have been prevented. B The absence of fire drills caused confusion among the trapped workers. C The Triangle Fire had a lasting impact on safety regulations. D Onlookers who witnessed the fire were horrified by what they saw.
Answers
Answer: A The Triangle Fire was a tragedy that could have been prevented.
Explanation:
The Triangle Fire was a tragedy that happened in the factory of Shirtwaist Company in New York City where there was.a.fore outbreak and about 145 employees of the company were killed.
The deaths could have been prevented because the company neglected safety measures such as having a good and reliable pump system, having readily available fire extinguishers. Also, what resulted in the lethal nature of the incident was because the doors within the building of the factory were locked.
The tragedy generated lots of attention and series of laws were been out in place in order to ensure that workers are safe at their workplace.
Calculate the pH of 0.046 M HClO4.a. 0.046b. 7.00c. 1.34d. 12.66e. none of these
Answers
The pH of a 0.046 M HClO₄ solution is (c) 1.34.
HClO₄, or perchloric acid, is a strong acid that dissociates completely in water. Given a 0.046 M solution of HClO₄, we can calculate its pH using the formula:
pH = -log₁₀[H⁺]
Since HClO₄ dissociates completely, the concentration of hydrogen ions [H⁺] in the solution will be equal to the concentration of the HClO₄, which is 0.046 M. Plugging this value into the formula:
pH = -log₁₀(0.046)
Calculating the logarithm gives us:
pH ≈ 1.34
Therefore, the pH of a 0.046 M HClO₄ solution is approximately 1.34. The correct answer is option (c).
Learn more about pH here: https://brainly.com/question/26424076
#SPJ11
IV - INTERPRETATION AND FOLLOW UP
TASK 6-explain the variation of concentrations as a function of time in the experiments.
TASK 7 - as the experiments proceeded, gypsum grains were also sampled and imaged with the aid of a Scanning Electron Microscope (Figure 3). The micrographs reveal a progressive coating of gypsum by newly formed crystals. In your opinion, what would be the next analytical steps to be pursued?
Answers
The concentration of gypsum in solution increased while that of sulfates decreased. The rate of gypsum precipitation decreased with time.
The experimental results revealed that the concentration of gypsum in solution increased with time, while that of sulfates decreased. This trend can be explained by the reaction between calcium and sulfate ions in the solution, which led to the precipitation of gypsum. The concentration of sulfate ions in solution decreased with time due to their consumption in the reaction.
As a result, the rate of gypsum precipitation decreased over time. The findings suggest that the reaction was not complete, and that a fraction of sulfate ions remained in solution. To investigate this further, one could perform additional experiments to measure the concentration of sulfate ions at various time points during the reaction.
In addition, one could study the effect of temperature, pH, and other parameters on the reaction rate and the properties of the gypsum crystals formed. Finally, one could compare the experimental results with theoretical models to gain a deeper understanding of the reaction kinetics and mechanisms.
Learn more about gypsum here:
https://brainly.com/question/18369612
#SPJ11
Calculate the mass of N in 2.34 g of N2H4?A) 4.68 g N B) 65.6 g N C) 28.02 g N D) 2.05 g N E) 2.34 g N
Answers
D) 2.05 g N. The molar mass of N2H4 is 32.045 g/mol. To calculate the mass of N in 2.34 g of N2H4, we need to first calculate the number of moles of N2H4:
moles of N2H4 = (mass of N2H4) / (molar mass of N2H4)
moles of N2H4 = 2.34 g / 32.045 g/mol
moles of N2H4 = 0.073 mol
Since there are two N atoms in one N2H4 molecule, we need to multiply the number of moles of N2H4 by 2 to get the number of moles of N:
moles of N = 2 x moles of N2H4
moles of N = 2 x 0.073 mol
moles of N = 0.146 mol
Finally, we can calculate the mass of N:
mass of N = (moles of N) x (molar mass of N)
mass of N = 0.146 mol x 14.007 g/mol
mass of N = 2.05 g
Find out more about molar mass
brainly.com/question/30892478
#SPJ11
Find the PEN for pb.

Answers
No of protons and no of electrons is 82 , no of neutrons is 125.
What is proton?Ernest Rutherford is father of Proton.It is a subatomic particle found in the nucleus of every atom. It has a positive electrical charge, equal and opposite to that of the electron.What is Neutron?It is a subatomic particle found in the nucleus of every atom except that of simple hydrogenIt derives its name from the fact that it has no electrical charge; it is neutralThe sum of total number of protons and total number of neutrons in the atomic nucleus yields the mass number of that atomic nucleusWhat is electron?It is a negatively charged subatomic particle that can be either bound to an atom or free It was discovered by the English physicist J.J. Thomson They are distributed around nuclei of atoms in atomic orbitals, which can be simply visualized as regions around the nucleus in which the probability of finding a specific electron is the highest.How to calculate PEN of an element?
PEN stands for proton, electron and neutron
Atomic number is equal to the number of protons (p) in an atom.Since atoms are neutral, it is also the same as the number of electrons (e) [ No of protons = no of electrons ] The atomic mass = number of protons + neutronsAn element is represented as ZXA,
where
Z = atomic number which is also equal to no of proton ( written on top of element)
A = atomic mass
So lead is represented as ⁸²Pb₂₀₇
proton: Since atomic number of lead is 82, it has 82 protonsElectron: It has 82 electrons, since atoms are neutral.Neutrons: no of neutrons = atomic mass - atomic number= 207 - 82 = 125
Learn more about PEN at: https://brainly.com/question/25254273
#SPJ13
The pressure on a 5.50 liter tank of anesthetic gas changes from 3.00 atm to 4.40 atm. What will be the new volume if the temperature remains constant?
Answers
The new volume of the gas at a pressure of 4.40 atm is approximately 3.75 liter
According to the ideal gas law, PV = nRT, the pressure and volume of a gas are inversely proportional to each other, assuming constant temperature. Therefore, if the pressure on a gas changes from P1 to P2 while the temperature remains constant, the new volume V2 can be calculated as:
V2 = (P1 x V1) / P2
where V1 is the initial volume of the gas.
Substituting the given values, we get:
V2 = (3.00 atm x 5.50 L) / 4.40 atm ≈ 3.75 L
For more question on volume click on
https://brainly.com/question/27100414
#SPJ11
The hoverboard is not the best way to get to school
Write a CER 3 evidence and 3 reasonings
Answers
The justification provides the "why" and "how" the evidence substantiates the assertion.
What is justification?Justification is defined as the act, procedure, or declaration that makes sinners righteous in God's eyes. The term "research justification" refers to the justification for conducting the research, which includes a description of the research's design and methodology.
Your students may offer the following justification: Matter is air (claim). When we added more air to the ball, we discovered that the weight of the ball rose (evidence). This demonstrates that weight, a property of matter, exists in air (reasoning).
Thus, the justification provides the "why" and "how" the evidence substantiates the assertion.
To learn more about justification, refer to the link below:
https://brainly.com/question/27795498
#SPJ1
Please help im almost out of time ill mark BRAINLIESTTT

Answers
Answer:
The first one is actually Location D
The second one is Location B
I can't read all of the 3rd one, but if it says winter, its B, if it says summer its D
Explanation: