In a boiling pot of water are a metal spoon and a wooden spoon of equal masses/size. Which spoon would likely be more painful (higher in temperature) to grab? Assume that both spoons have been in the same pot of boiling water for the same amount of time. Explain this phenomena using the following terms: Heat, Mass, Temperature, Specific Heat Capacity, Heat Flow. Consider all possible factors in your explanation
Answers
The metal spoon is hotter than the wooden spoon due to its higher mass,
Heat is the energy transferred from one body to another due to a temperature difference. The amount of heat transferred is proportional to the mass of the object and its specific heat capacity. Specific heat capacity is the amount of heat required to raise the temperature of one unit mass of the substance by one degree Celsius.
In this scenario, the two spoons are of equal size, but the metal spoon has a higher mass and specific heat capacity compared to the wooden spoon. When both spoons are placed in the boiling water, heat flows from the water to the spoons until they reach the same temperature as the water.
However, due to the higher mass and specific heat capacity of the metal spoon, it requires more heat energy to raise its temperature compared to the wooden spoon. As a result, the metal spoon takes a longer time to reach the same temperature as the wooden spoon.
Additionally, metals are better conductors of heat compared to wood. Therefore, the metal spoon conducts the heat more efficiently from the boiling water to the handle, making it hotter than the wooden spoon.
Overall, the metal spoon is hotter than the wooden spoon due to its higher mass, higher specific heat capacity, and better heat conduction properties. This is why it would be more painful to grab.
Related Questions
Calcium carbonate, CaCO_3, decomposes upon heating to calcium oxide and carbon dioxide. What mass of solid calcium carbonate is required to produce 2.40 liters of carbon dioxide measured at Standard Temperature and Pressure (STP)?
(A) 10.7 g
(B) 21.4 g
(C) 50.0 g
(D) 100. g
Answers
The mass of solid calcium carbonate is required to produce 2.40 liters of carbon dioxide measured at Standard Temperature and Pressure (STP) is (C) 50.0 g.
Let's learn this step-by-step:
Using stoichiometry, we can determine the mass of CaCO3 needed to form 2.40 L CO2 at STP (standard temperature and pressure).
Since calcium carbonate decomposes into calcium oxide and carbon dioxide as below;
CaCO3(s) → CaO(s) + CO2(g)
We can write the stoichiometry equation of calcium carbonate to carbon dioxide as shown below.
CaCO3(s) → CaO(s) + CO2(g)
1 mol → 1 mol of CO2
Since at STP; 1 mole of gas = 22.4 L
Therefore, 1 mol of CO2 has a volume of 22.4 L at STP.2.40 L of CO2 is therefore;
2.40 L/22.4 L/mol = 0.107 mol CO2
From the equation above,
1 mol of CaCO3 → 1 mol CO20.107 mol CO2 → 0.107 mol CaCO3
The molar mass of CaCO3 is 100 g/mol
0.107 mol CaCO3 → 0.107 x 100 g/mol = 10.7 g of CaCO3 is required to form 2.40 L of CO2 at STP.
Therefore, the answer is option (A) 10.7 g.
Learn more about stoichiometry: What is stoichiometry in chemistry? https://brainly.com/question/14935523
#SPJ11
fill in the blanks with the words given below-
[Atoms, homogeneous, metals, true, saturated, homogeneous, colloidal, compounds, lustrous]
1.An element which are sonorous are called................
2.An element is made up of only one kind of ....................
3.Alloys are ............................. mixtures.
4.Elements chemically combines in fixed proportion to form ........................
5. Metals are................................... and can be polished.
6. a solution in which no more solute can be dissolved is called a .................... solution.
7. Milk is a .............. solution but vinegar is a .................. solution.
8. A solution is a ................... mixture.
pls help, could not get these answers
Answers
Answer:
1. Metal.
2. Atom.
3. Homogeneous
4. Compounds.
5. Lustrous
6. Saturated.
7. Colloidal; true.
8. Homogeneous.
Explanation:
1. An element which are sonorous are called metal.
2. An element is made up of only one kind of atom.
3. Alloys are homogeneous mixtures.
4. Elements chemically combines in fixed proportion to form compounds.
5. Metals are lustrous and can be polished.
6. A solution in which no more solute can be dissolved is called a saturated solution.
7. Milk is a colloidal solution but vinegar is a true solution.
8. A solution is a homogeneous mixture.
4. Show the completion of the following equations: a) CH₂C CH₂C b) CH₂C CH₂C O O + NH3 O O NH2 200°C. NH₂ O A
Answers
a) CH₂C=CH₂ + C (triple bond) CH₂
b) CH₂C=CH₂ + O (double bond) O + NH₃ → O (double bond) O NH₂ + NH₂
In the given equations, we are asked to show the completion of the reactions. Let's break down each equation separately:
a) CH₂C=CH₂ + C (triple bond) CH₂:
The reactant in this equation is CH₂C=CH₂, which is an alkene. By adding a carbon atom with a triple bond to the molecule, the reaction is completed. The product is C (triple bond) CH₂, representing a terminal alkyne.
b) CH₂C=CH₂ + O (double bond) O + NH₃ → O (double bond) O NH₂ + NH₂:
In this equation, we start with CH₂C=CH₂, an alkene, and add O (double bond) O and NH₃ to complete the reaction. The result is O (double bond) O NH₂, representing a carbamate, and NH₂, indicating the presence of an amino group.
In summary, the completion of the given equations results in the formation of a terminal alkyne (C≡CH₂) in the first case and a carbamate (O=C(ONH₂)₂) along with an amino group (NH₂) in the second case.
Learn more about Alkene
brainly.com/question/30217914
#SPJ11
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2?
Answers
If you started with 1.5 moles of H2 at STP, you would have approximately 33.6 liters of volume of hydrogen (H₂) gas.
What is the volume of the hydrogen gas at STP?
To determine the number of liters of H2 you have, we need to consider the conditions under which the gas is being held (i.e. temperature and pressure), as well as the molar volume of H2 at those conditions.
At standard temperature and pressure (STP), which is 0°C (273.15 K) and 1 atm (101.325 kPa), the molar volume of any ideal gas is approximately 22.4 L/mol.
Therefore, at STP, 1.5 moles of H₂ would occupy approximately:
V = n x Vm = 1.5 mol x 22.4 L/mol = 33.6 L
Learn more about volume of gas here: https://brainly.com/question/25736513
#SPJ1
The complete question is below:
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2? (assume STP condition)
synthesis of acetaminophen from p-aminophenol and acetic anhydride mechanism
Answers
The synthesis of acetaminophen from p-aminophenol and acetic anhydride involves an acylation reaction. Here is a simplified mechanism for the synthesis:
Step 1: Protonation of the p-aminophenol: In the presence of an acid catalyst, such as sulfuric acid (H2SO4), the p-aminophenol molecule is protonated, resulting in the formation of the p-aminophenol cation (p-AP+). Step 2: Formation of the acylium ion: Acetic anhydride is then added to the reaction mixture. The acetic anhydride molecule loses one of its oxygen atoms, forming an acylium ion (CH3CO+). Step 3: Nucleophilic attack: The lone pair of electrons on the nitrogen atom of the p-aminophenol cation (p-AP+) acts as a nucleophile and attacks the electrophilic carbon of the acylium ion (CH3CO+). This results in the formation of a new bond between the nitrogen atom and the carbon atom of the acylium ion, while one of the oxygen atoms of the acylium ion is displaced.Step 4: Proton transfer: A proton transfer occurs from the oxygen atom of the acylium ion (now attached to the nitrogen atom) to a nearby molecule or solvent, regenerating the aromaticity of the phenyl ring. Step 5: Tautomerization: The resulting intermediate undergoes tautomerization, where a hydrogen atom is transferred from the hydroxyl group to the adjacent nitrogen atom. This forms the final product, acetaminophen.
To learn more about synthesis;
https://brainly.com/question/30514814
#SPJ11
can someone help me please
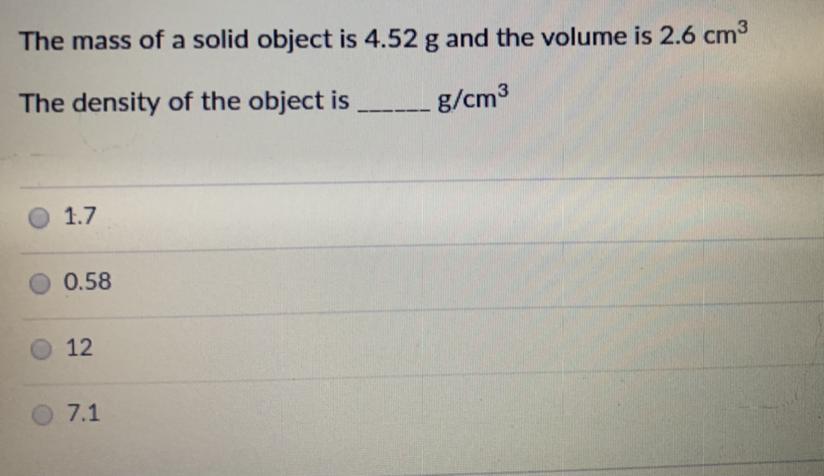
Answers
Based on the balanced equation : 2 K + Cl2 → 2 KCl If you start with 4 K, how many KCl can you make?
Answers
The law of conservation of mass is obeyed by a balanced chemical equation. The number of moles of KCl produced from 4K is 4KCl.
What is a balanced chemical equation?An equation in which the amount of the reactants and products on both sides of the reaction are equal is defined as the balanced chemical equation. In a balanced equation the number of atoms of each element is same on both sides.
According to the law of conservation of mass, mass can neither be created nor be destroyed. All the balanced chemical equation satisfies this law.
Here when 4K is used the amount of KCl produced is given as:
4K + 2Cl₂ → 4KCl
Here there are 4 'K' atoms and 4 'Cl' atoms. Hence the number of the respective atoms are equal on both sides of the equation.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
Someone please help !

Answers
The _____
includes the top portion of Earth's crust, all the waters that cover
Earth's surface, and the surrounding atmosphere.
Answers
Answer:
biosphere
You can even double check on g00gle.
When you are finding core electrons, do you count only the first inside shell or all the inside shells?

Answers
Answer:
Valence electrons are the electrons orbiting the nucleus in the outermost atomic shell of an atom. Electrons that are closer to the nucleus are in filled orbitals and are called core electrons
Explanation:
yup
Alex built a model to show how light bends when placed into water. To do this, Alex filled a fish tank up with water, and shined a green laser through the water. The angle he shined the laser into the fish tank is the line above the water, and the angle that the light bent is seen below the water. What is a limitation of this model? The model cannot show how light bends. The model is unable to use different colors of light The model cannot calculate the speed of light The model is not 3 dimensional
Answers
Answer:
The model cannot show how light bends.
Explanation:
which of these statements is incorrect? a. the binding forces in a molecular solid include london dispersion forces. b. ionic solids are insulators. c. all of the statements (a-d) are correct. d. molecular solids have high melting points. e. ionic solids have high melting points.
Answers
Ionic solids are therefore insulators, which is the right response. Choice "B."
Explain what an insulator is.An insulator is any substance that prevents the energy—such as electricity, heat, or cold—from the moving through it easily. Good insulators include glass, plastic, rubber, and the wood.
Which insulator is the used most frequently?The most popular and generally accessible type of insulation, blanket, comes in the form of rolls or batts. Flexible fibres, most frequently fibreglass, make up this substance. Additionally, natural fibres like cotton and sheep's wool are available in batts and rolls, along with artificial fibers and mineral wool.
To know more about insulators visit:
https://brainly.com/question/24909989
#SPJ4
A gas with a volume of 250 mL at a temperature of 293K is heated to 324K. What is the new volume of the gas?
Answers
Answer:
you can simply answer vl/t1=v2/t2
Which of the following statements is wrong about cathode rays?A.They travel in straight lines towards cathodeB.They produce heating effectC.They carry negative chargeD.They produce X-rays when strike with material having high atomic masses
Answers
The statements wrong about cathode rays is none of the given option. All the given option is true about the cathode rays.
The statements for the cathode rays is given as :
A. They travel in straight lines towards cathode. This statement is true about the cathode rays.
B. They produce heating effect. This statement is true about the cathode rays.
C. They carry negative charge. This statement is true about the cathode rays.
D. They produce X-rays when strike with material having high atomic masses. This statement is true about the cathode rays.
Thus, none of the option is incorrect about the cathode rays.
To learn more about cathode rays here
https://brainly.com/question/26500956
#SPJ4
which gas is plotted using the y-axis on the right?which gas is plotted using the y-axis on the right?methane (ch4)carbon dioxide (co2)nitrous oxide (n2o)the total of all three gases
Answers
Without having access to the specific graph you are referring to, I cannot definitively determine which gas is plotted using the y-axis on the right. However, the gases you mentioned are methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O). It is possible that the graph shows the total of all three gases as well. Please refer to the graph's labels or legend for clarification on which gas is plotted on the right y-axis.
To know more about graphs of gases visit;
https://brainly.com/question/12092119
#SPJ11
Classify each of these solids as ionic, molecular, metallic, or covalent (also known as covalent-network solids or macromolecular solids). Ionic Molecular Metallic Covalent Answer Bank cal, C (diamond) AICI,
Answers
The given solids are classified as: Diamond- covalent solid, aluminum chloride-Ionic solids, Copper- Metallic solids, dry ice- molecular solids
The classification of a solid depends on the type of chemical bonding between the atoms or molecules that make up the solid. Covalent solids are held together by covalent bonds, ionic solids by ionic bonds, metallic solids by metallic bonds, and molecular solids by intermolecular forces between the molecules.
Some other examples of such solids are: Covalent solids- Graphite, silicon dioxide (quartz), silicon carbide. Ionic solids- Sodium chloride, magnesium oxide, calcium carbonate, potassium iodide. Metallic solids- Iron, gold, aluminum. Molecular solids- sulfur, ice (solid water), solid nitrogen
To know more about solids here
https://brainly.com/question/30333708
#SPJ4
The complete question is:
Classify each of these solids as ionic, molecular, metallic, or covalent (also known as covalent-network solids or macromolecular solids).
Diamond, Aluminum chloride, copper, dry ice.
Which elements have the most similar chemical properties? A. Si, As, and Te B. Mg, Sr, and Ba C. N2, O2, and F2 D. Ca, Cs, and Cu
Answers
The elements that have the most similar chemical properties are; N₂, O₂, and F₂. Option C is correct.
Nitrogen (N), oxygen (O), and fluorine (F) are all members of Group 16 (also known as Group VIA or Group 6A) of the periodic table. These elements are known as the chalcogens and share similar chemical properties due to their similar electron configurations and valence electron arrangements.
Nitrogen (N) has the electron configuration 1s₂ 2s₂ 2p₃, oxygen (O) has the electron configuration 1s₂ 2s₂ 2p₄, and fluorine (F) has the electron configuration 1s₂ 2s₂ 2p₅. They all have a tendency to gain electrons to achieve a stable noble gas electron configuration.
These elements readily form covalent bonds with other elements, particularly hydrogen, carbon, and other nonmetals. They are known for their reactivity and ability to form compounds such as nitrogen oxides (NOx), oxygen compounds (such as water and oxides), and fluorides.
Hence, C. is the correct option.
To know more about elements here
https://brainly.com/question/24407115
#SPJ4
Below is a diagram showing the chemical energy (Ech) for the formation of water. Is the reaction exothermic or endothermic?

Answers
Answer: exothermic
Explanation: chemical reactions that release energy is called exothermic, and in this case, more energy is being released. (I THINK)
The reaction shown in the picture is exothermic reaction.
What is exothermic reaction?The exothermic reaction leads to the release of energy. This energy is often higher than the combined energy of the reactants.
It can be understood that the net amount of energy required to initiate an exothermic reaction is less than the net amount of energy released by the reaction. When a calorimeter, a device used to measure the heat released by a chemical reaction, the net amount of heat energy that flows through the device is equal to the negative of the total energy change of the system.
In the picture, first reactants had high energy and after the formation of the product the energy decreases.
The nuclear fission of one atom of uranium-235 releases more than 2.5 million times the energy that is produced from the combustion of coal.
The fission of one atom can cause a chain reaction in which the neutron from the fission of one uranium-235 atom can strike another atom, leading to its nuclear fission.
Therefore, The reaction shown in the picture is exothermic reaction.
Learn more about exothermic reaction, here:
https://brainly.com/question/10373907
#SPJ2
true or false: a hydrate is a solid ionic compound (or, a salt) in which water molecules are associated with the ions of the compound.
Answers
The statement is true because, A hydrate is a solid ionic compound (or salt) in which water molecules are associated with the ions of the compound.
These water molecules form hydration shells around the ions, forming a lattice structure that helps stabilize the compound.
The hydration shells, which are composed of water molecules, surround the ions of the compound and form a lattice structure. This structure helps to stabilize the compound, as the water molecules attract the ions and effectively bond them together.
Additionally, the hydration shells can also help to protect the ions from reacting with other molecules, preventing the compound from being broken down into its component elements.
Learn more about hydrate :
https://brainly.com/question/25820990
#SPJ4
Which of the greenhouse gases are carbon compounds?
Answers
Answer:
Methane (CH4),Carbondioxide green house gases are carbon compounds
Carbon dioxide (CO2) and methane (CH4) are two powerful greenhouse gases produced by the carbon cycle.
A waste management specialist for a city is designing a recycling program to reduce the amount of synthetic materials that enter the local landfill. Which of the following criteria would be best for the specialist's design? A. to make it easy for the residents of the city to recycle synthetic materials B. to keep anything made of natural materials from entering the landfill C. to make residents travel a long distance to recycle synthetic materials D. to keep residents from using anything made of synthetic materials
Answers
Option A would be the right recycling program to reduce the number of synthetic materials that enter the local landfill. Hence option A would be the suitable criteria for the specialist's design.
Option A is correct because, with the providence of the knowledge to recycle synthetic materials, there will be less waste accumulation. Hence most synthetic materials can be expected to be recycled.
Option B is wrong because restricting natural materials from entering the field will further increase the use of synthetic materials. Hence there will be more accumulation of waste.
Option C is wrong because making the residents travel long distances will be tiresome, and the outcome of disposing of them will be little.
Option D is wrong because synthetic materials are valuable in various manufacturing and widely used in our daily lives. Hence we will lose the potential benefits.
Learn more about waste management at,
https://brainly.com/question/3406415
A photon of wavelength 1,094 nm is emitted when an electron in hydrogen makes a transition to the third level. determine the level that the electron started it.
Answers
The electron started in the second energy level (n₁ = 2) before transitioning to the third level.
To determine the initial level of the electron in a hydrogen atom, we can use the Rydberg formula, which relates the wavelength of a photon emitted or absorbed during an electron transition to the energy levels in hydrogen:
1/λ = R * (1/n₁² - 1/n₂²)
Where, λ is the wavelength of the photon,
R is the Rydberg constant (approximately 1.097 x 10^7 m^-1),
n₁ is the initial energy level,
n₂ is the final energy level.
Given that, the wavelength of the emitted photon is 1,094 nm (or 1.094 x 10^-6 meters) and the electron transition occurs to the third level (n₂ = 3), we can substitute these values into the formula and solve for n₁:
1/λ = R * (1/n₁² - 1/n₂²)
1/(1.094 x 10^-6) = 1.097 x 10^7 * (1/n₁² - 1/3²)
Simplifying the equation:
1.094 x 10^6 = 1.097 x 10^7 * (1/n₁² - 1/9)
1/n₁² - 1/9 = (1.094 x 10^6) / (1.097 x 10^7)
1/n₁² - 1/9 ≈ 0.0997
1/n₁² ≈ 0.0997 + 1/9
1/n₁² ≈ 0.1997
n₁² ≈ 1 / 0.1997
n₁² ≈ 5.004
n₁ ≈ √5.004
n₁ ≈ 2.24
Therefore, the electron started in the second energy level (n₁ = 2) before transitioning to the third level.
Learn more about electron from the given link:
https://brainly.com/question/26084288
#SPJ11
If 2 isotopes of Osmium existed, Os-190 and Os-191, which one would have the highest percent abundance? How do you know?
Answers
If 2 isotopes of Osmium existed, Os-190 and Os-191, one would have the highest percent abundance is Os - 190.
The isotopes are elements of the same family that all have the same number of protons but have the different number of neutrons. The two common type of isotopes of osmium is Os - 190 and the Os - 191. The isotopes whose mass is closer to the the relative atomic mass of osmium would have the highest percentage of the abundance. that is osmium 190.
Thus, If 2 isotopes of Osmium existed, Os-190 and Os-191, one would have the highest percent abundance is Os-190.
To learn more about isotopes here
https://brainly.com/question/24707516
#SPJ1
Describe and give and example of each of the following types of solutions: 1. alloy 2. liquid solution 3. gaseous solution
Answers
An alloy is a solid solution of metals or a metal and a non-metal. A liquid solution is a homogeneous mixture where the solvent is a liquid, and a gaseous solution is a homogeneous mixture of gases.
An alloy is formed when two or more metals, or a metal and a non-metal, are combined to create a solid solution. The atoms of the different elements mix together at the atomic level, resulting in a homogeneous material with unique properties. For example, bronze is an alloy composed of copper and tin. It possesses enhanced strength and durability compared to its individual components, making it suitable for applications like statues and musical instruments.
A liquid solution consists of a solvent, which is a liquid, and one or more solutes dissolved within it. The solutes can be solid, liquid, or gas. When the solute particles disperse evenly throughout the solvent, a homogeneous mixture is formed. One common example is saltwater, where sodium chloride (the solute) dissolves in water (the solvent) to create a liquid solution.
A gaseous solution is a homogeneous mixture of two or more gases. The gases mix at the molecular level, creating a uniform distribution of gas particles. An example of a gaseous solution is air, which is primarily composed of nitrogen, oxygen, carbon dioxide, and other trace gases. The different gases in air mix thoroughly, resulting in a well-blended gaseous solution.
To learn more about solvent click here:
brainly.com/question/11985826
#SPJ11
A. Mechanical waves
B. Infrared waves
C. Light waves
D. Electromagnetic waves

Answers
uwu
What coefficient is needed to balance this equation? ____Fe + 4H2O --> Fe6O4 + 4 H2 *
Answers
Answer:
the answer is 1
the coefficient needed to balance this equation is 1
Explanation:
What is the relationship among the efficieny output work and input work of a machine?
Answers
The efficiency of a machine is the ratio of the output work it performs to the input work required to operate it. This ratio is expressed as a percentage, and it tells you how much of the input work is converted into useful output work.
For example, if a machine requires 10 units of input work to perform 5 units of output work, its efficiency would be 50%. This means that 50% of the input work is converted into useful output work, while the remaining 50% is lost as waste heat, friction, or other forms of energy dissipation.
In general, the efficiency of a machine is determined by its design, as well as the materials and components used to construct it. Machines that are well-designed and constructed with high-quality materials will typically have higher efficiencies than those that are poorly designed or made with lower-quality materials.
It's also important to note that the efficiency of a machine can vary depending on the type and amount of work it is performing. For example, a machine may be more efficient at performing certain tasks than others, or it may become less efficient as it wears out or is subjected to heavy use.
Balancing Chemical Reactions Worksheet A glow stick is a popular toy and safety device. To use a glow stick, you bend a small flexible plastic tube to break a small glass capsule inside, at which point the stick begins to glow. How do you think this works
Answers
Answer:
Concept of chemi-fluorescence
Explanation:
A glow stick usually consists of two chemicals in a larger plastic tube: , a base catalyst (mostly sodium salicylate), and a suitable dye (sensitizer, or fluorophor). This creates an exergonic reaction when mixed together.
When a glow stick is bent, the flurophor which is a chemical that easily re-emits light upon excitation in smaller capsules is released into the other substance, there by causing it to emit radiation/light in the uv-visible region. The brightness and longevity of the glow stick is determined by varying the concentration of these chemicals.
I hope this explanation clarifies things.
A ball initially at rest is dropped from a window sill 14.2 m off the ground. Find the velocity of the ball the moment it hits the ground. Round your answer to 2 decimals places.
Answers
Answer:
16.68 m/s
Explanation:
The following data were obtained from the question:
Height (h) = 14.2 m
Initial Velocity (u) = 0 m/s
Final velocity (v) =?
Thus, the velocity (v) with which the ball hit the ground can be obtained as follow:
v² = u² + 2gh
NOTE: the acceleration due to gravity (g) = 9.8 m/s²
v² = u² + 2gh
v² = 0² + 2 × 9.8 × 14.2
v² = 0 + 278.32
v² = 278.32
Take the square root of both side
v = √278.32
v = 16.68 m/s
Therefore, the velocity with which the ball hit the ground is 16.68 m/s
There are two isotopes of chlorine. The lighter one with a mass number of 35 (Cl- 35) and the heavier Cl - 37. The atomic mass of chlorine is 35.45 u. Given the mass of chlorine isotopes and the atomic mass of chlorine, determine which isotope is more. Justify your answer.
i need help asap, pls respond quick
Answers
Answer:
Cl-35 isotope is more abundant.
Explanation:
How to calculate the abundance of isotopes in a mixture from the mass of isotopes and the average atomic mass of the element?
The atomic mass of an element having two or more naturally occurring isotopes is calculated using the following relation : Average atomic mass = % abundance of isotope A x atomic mass of isotope A + % abundance of isotope B x atomic mass of isotope B.Solution :
Say the % abundance of Cl - 35 is x, i.e, 100 units of Cl contains x units of Cl-35.
Therefore, the % abundance of Cl - 37 is (100 - x).
∴ [35 x + 37 (100-x)] = 35.45 x 100
Simplifying the above equation, we get
-2x + 3700 = 3545
Subtracting 3700 from both sides of the equation, we get
-2x = -155
or, 2x = 155
Dividing both sides of the equation by 2, we get
x = 155 ÷ 2 = 77.5
∴ 100 -x = 22.5
Thus, Cl-35 is more abundant (77.5%) than Cl-37 (22.5%).
To know more about isotopic abundance, visit:
https://brainly.com/question/24873591