Noble gases are unreactive because, except for helium, they have a stable arrangement of____ valence electrons in the outer energy level. This arrangement is called a(n)____
Answers
Noble gases are unreactive because, except for helium, they have a stable arrangement of eight valence electrons in the outer energy level. This arrangement is called a(n) octet.
The octet rule states that atoms tend to gain, lose or share electrons in order to achieve a stable octet arrangement, similar to that of noble gases. Since noble gases already have a stable octet, they have no need to form chemical bonds with other elements.
Noble gases are unreactive because, except for helium, they have a stable arrangement of 8 valence electrons in the outer energy level. This arrangement is called a(n) full or complete electron shell.
To learn more about octet rule click here
brainly.com/question/865531
#SPJ11
Related Questions
A microwave oven has a power rating of 850 W.
If the microwave oven is turned on for 6 minutes, how much energy will use? Give me the simplest answer possible as for a yr6-8 student to understand.. please..
Answers
Answer:
Explanation:
Before we jump into it, I just want to point out that a microwave oven has two
power ratings. One is the amount of electrical power it takes from the wall outlet to do its job. The other is the "cooking power" it delivers into the chamber in the form of microwave radiation to heat the meatloaf. The utility power required from the wall outlet is always more than the cooking power radiated into the chamber.
But we didn't really need to go into all that in order to answer the question.
1 watt = 1 joule per second
1 minute = 60 seconds
(850 watts) x (1 (joule/sec) / watt) x (60sec/min) x (6 minutes) = 306,000 joules
A substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called.
Answers
A substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called acid.
What is an acid?An acid is a substance that produces hydrogen ion as the only positive ion produced in water. This is the Arrhenius definition of an acid. As such, the acid gives out a proton to water and oxonium ion is formed.
Thus, substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called acid.
Learn more about acid:https://brainly.com/question/14072179
#SPJ4
Water (2) is a/an _______________ compound.
Answers
.Which of the following metals will dissolve in HCl?
(a) Ca
(b) Na
(c) Cd
(d) Ni
(e) all of the above
Answers
Hydrochloric acid (HCl) is a strong acid that can dissolve many metals, including calcium (Ca), sodium (Na), cadmium (Cd), and nickel (Ni).
The correct answer is E.
When these metals come into contact with HCl, they undergo a chemical reaction in which they are oxidized and dissolved.The reaction between HCl and metals such as Ca, Na, Cd, and Ni is exothermic and can release hydrogen gas (H2).
This reaction is often used in laboratory experiments and industrial processes to dissolve metals or to generate H2 gas. However, it is important to handle HCl with caution as it is a corrosive and toxic substance that can cause burns and respiratory problems if not used properly.
To Know more about Hydrochloric visit;
https://brainly.com/question/20030736
#SPJ11
If the pk values for phosphoric acid are 2. 15, 6. 82 and 12. 38, at what ph would one observe equal amounts of h2po4- and hpo42-?
Answers
Answer: https://homework.study.com/explanation/if-the-pk-a-values-for-phosphoric-acid-are-2-15-6-82-and-12-38-at-what-ph-would-one-observe-equal-amounts-of-h-2-po-4-and-hpo-4-2.html
Explanation:
website to the answer lol
Someone help me quick! Will give brainliest
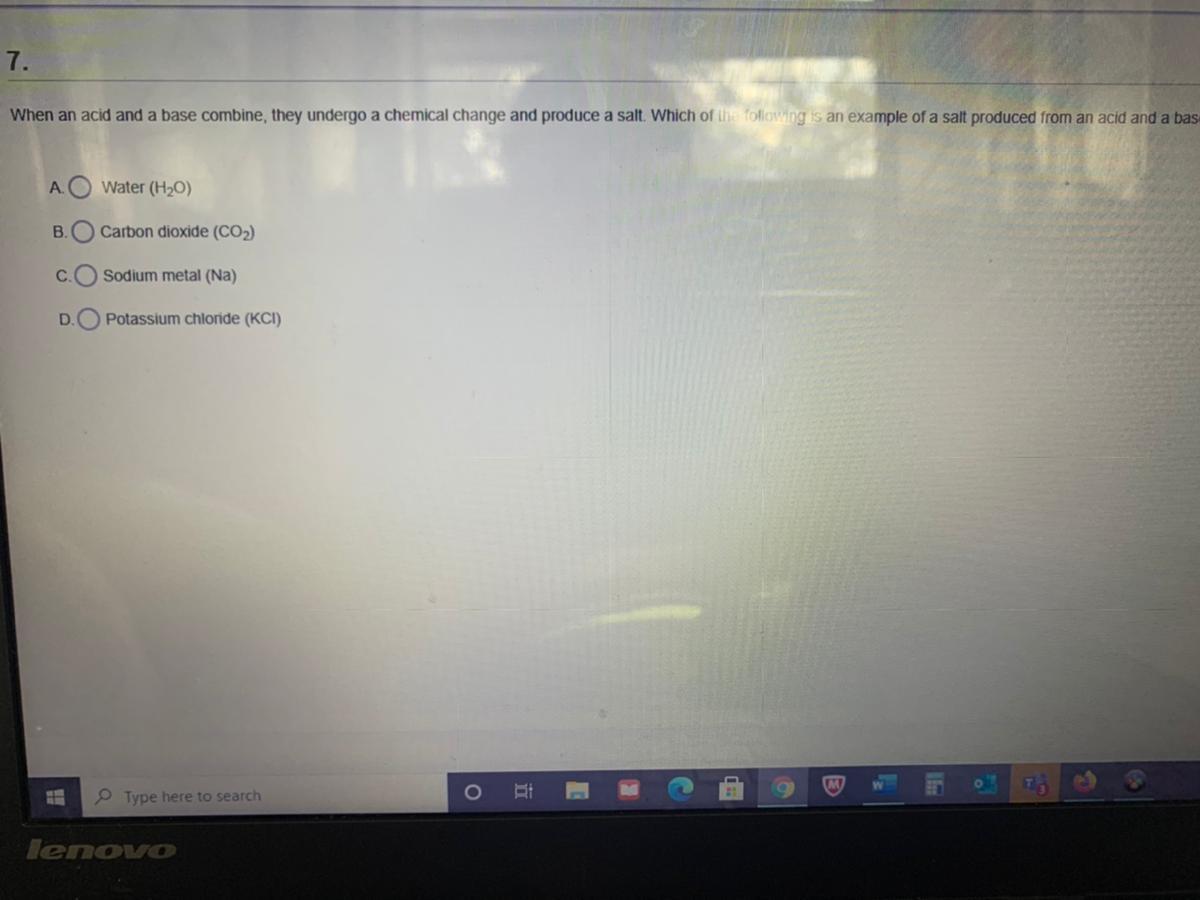
Answers
Answer:
WHY DO YOU NEED HELP HUH
DON'T CHEAT READ AND STUDY
Explanation:
Answer:
It's C
Explanation:
Will nuclear fusion be used for power on other planets?.
Answers
Answer: Hope that helps!
Explanation: Researchers at NASA and the Department of Energy recently tested key technologies for developing a nuclear fission reactor that could power a human outpost on the moon or Mars. The tests prove that the agencies could build a “safe, reliable, and efficient” system by 2020, the year NASA plans to return humans to the moon.
This Venn diagram is used to compare the products and reactants of a reaction of baking soda and vinegar. What could be put into the intersection? Precipitates Chemical compounds Solid compounds Gases
Answers
I think and I'm sure it is gases
Answer:
gasses
Explanation:
i took the quiz
A chemist has 2. 0 mol of methanol (CH3OH). The molar mass of methanol is 32. 0 g/mol. What is the mass, in grams, of the sample? 16 grams 30 grams 32 grams 64 grams.
Answers
The mass, in grams, of the sample of methanol (CH₃OH) is 64 grams.
How we calculate mass from moles?Mass of any substance can be calculated by using moles as:
n = W/M, where
W = required mass
M = molar mass
In the question that:
Moles of methanol = 2mole
Molar mass of methanol = 32g/mole
On putting these values in the above equation, we get
W = n × M
W = 2mole × 32g/mole = 64g
Hence, 64 grams is the mass of the sample.
To know more about moles, visit the below link:
https://brainly.com/question/15374113
if 2Na + Cl2 --> 2NaCL ... what happened to the two on Cl? and if 2H2O --> 2H2 + O2 what happened to the 2 on the O as well? could you please answer this in like sort of a simple way? my brain is mush at the moment lol.
Answers
Explanation:
when a big number is behind a compound, it means there is 2 of it.
for example 2Na would mean there is a sodium atom and another sodium atom not joined together, but they're just near.
when there is a 2 (which should be small and at the bottom) after an atom, it means there are 2 of that atom.
i hope this helps

how many carbon dioxide molecules are produced if 8.45 x 1023 of water molecules of water are produced
Answers
The given question is incomplete. The complete question is:
Determine how many carbon dioxide molecules are produced if molecules of water are produced
\(C_2H_6(g)+O_2 (g)\rightarrow CO2(g)+H_2O(g)\)
Answer: \(5.60\times 10^{23}\) molecules of carbon dioxide are produced
Explanation:
The balanced chemical equation is:
\(2C_2H_6(g)+7O_2 (g)\rightarrow 4CO2(g)+6H_2O(g)\)
\(\text{Moles of water}=\frac{\text{given molecules}}{\text{Avogadros number}}=\frac{8.45\times 10^{23}}{6.023\\times 10^{23}}=1.40moles\)
Accoding to stoichiometry:
6 moles of water are produced along with = 4 moles of carbon dioxide
Thus 1.40 moles of water are produced along with = \(\frac{4}{6}\times 1.40=0.93\) moles of carbon dioxide
Molecules of carbon dioxide = \(moles\times {\text {Avogadros number}}=0.93\times 6.023\times 10^{23}=5.60\times 10^{23}\)
The volume of container 2 i 27. 32 L. How many mole of the ga are in container 2?
Answers
The number of moles in container 2 is 33.3moles when the container has 27.32L of gas inside it
The number of moles of gas in container 2 can be calculated using the Ideal Gas Law:
n = PV/RT
where n is the number of moles of the gas with known volume,
P is the pressure (assumed to be 1 atm for ideal gases),
V is the volume (27.32 liters),
R is the ideal gas constant (0.0821 L·atm/mol·K) and
T is the temperature (assumed to be 273.15 K).
Plugging in the values, we get:
n = (1 atm)(27.32 L)/(0.0821 L·atm/mol·K)(273.15 K)
n = 33.3 mol
Therefore, there are 33.3 moles of gas in container 2.
To know more about gas laws in chemistry, click below:
https://brainly.com/question/25290815
#SPJ4
When elements form ionic bonds, each atom is left with a __________________ outer shell.
WILL MAKE BRAINLIEST !!! help plssss
Answers
Answer:
Each atom is left with a complete outer shell. An ionic bond forms between a metal ion with a positive charge and a nonmetal ion with a negative charge.
HOPE DAT HELPS BRO!
Can someone help me with this i don't understand it.

Answers
Answer:
The subscript next to the letter "C" will always tell you the number of carbon atoms.
PLEASE HELP ME !
Electromagnetic waves will travel the SAME SPEED (speed of light) WITHIN a medium as it does WITHOUT a medium. Question 4 options: True False
Answers
Answer:
False
Explanation:
Emectromagnetic waves are slowed down by even the most transparent of mediums.
explain why carbon monoxide is formed when some of the air holes in a water heater get blocked ?

Answers
Select the correct answer.
Which statement best describes how chemical equations demonstrate conservation of mass?
A.
The number of reactants is the same as the number of products.
B.
The compounds are the same on each side of the reaction.
C.
The number of atoms of each element is the same on each side of the equation.
D.
The state of matter is the same on each side of the equation.
Answers
Answer:
All of the Above
A.The number of reactants is the same as the number of products.
B.The compounds are the same on each side of the reaction.
C.The number of atoms of each element is the same on each side of the equation.
D.The state of matter is the same on each side of the equation.
Explanation:
Answer:
C
Explanation:
I did the test
1. 204x1024 atoms of Calcium would weigh how many grams?
Answers
A, 1.204x10²⁴ atoms of calcium would weigh approximately 8.014 grams.
To determine the weight in grams of 1.204x10²⁴ atoms of calcium, we need to use the molar mass of calcium and Avogadro's number.
The molar mass of calcium (Ca) will be approximately 40.08 g/mol. This will be found on the periodic table.
Avogadro's number (NA) is approximately 6.022 x 10²³ atoms/mol. It will represents the number of atoms or molecules in one mole of the substance.
Now we can calculate the weight in grams using the given number of atoms;
weight = (number of atoms) x (molar mass) / Avogadro's number
weight = (1.204x10²⁴ atoms) x (40.08 g/mol) / (6.022 x 10²³ atoms/mol)
Simplifying the calculation;
weight = (1.204 x 40.08) / 6.022 g
weight ≈ 48.255 / 6.022 g
weight ≈ 8.014 g
Therefore, 1.204x10²⁴ atoms of calcium would weigh approximately 8.014 grams.
To know more about calcium here
https://brainly.com/question/32451675
#SPJ4
what is the first thing you do in any conversion question?
Answers
Answer:
You confirm the units involved, which would make you to understand the factors required.
Explanation:
Conversion of units is a process in which a given unit of a physical quantity is accurately transformed into another. Example, when the mass of a substance in grams is converted to kilograms. Or conversion of a given quantity of substance from liters to gallons.
When conversion is required, the first thing to do is to determine the units involved. That is, which unit do you want to convert to the required unit. This would give the understanding of the factors that are needed.
facell's surface area-to-volume ratio increased from 3:1 to 4:1, what impact would that have on the transport of materials across the cell membrane? A. It would be unchanged B. transport would increase C. transport would decrease D. none of the above
Answers
Answer:
Concentration gradient, size of the particles that are diffusing, and temperature of the system affect the rate of diffusion. Some materials diffuse readily through the membrane, but others require specialized proteins, such as channels and transporters, to carry them into or out of the cell.
Explanation:
Hopefully this helps :)
The Planck constant can be written as 6.626070 x 10-34
Choose the correct exponential notation for Planck's constant
expressed with three significant figures.
a 6.62 x 1034 J.S
b 6.63 x 10-34 j.s
C 6.02 x 1034 J.S
Answers
The correct exponential notation for Planck's constant is\(6.63\times10^{-34} Js\).
Explanation:
Given that,
Planck constant =\(6.626070\times10^{-34} Js\)
We need to find the correct exponential notation for Planck's constant
According to the given options,
In option (a) :
The given value in option is \(h= 6.62\times10^{34} J.s\)
In this option, the given value is in three significant figures but the negative sign is missing in the exponential term.
So, this is the incorrect option.
In option (b) :
The given value in question is \(6.626070\times10^{-34} Js\)
We write the value of the Planck constant with three significant figures.
The Planck constant with three significant figures will be
\(h=6.63\times10^{-34} Js\)
So, this is the correct option.
In option (c) :
The given value is \(h= 6.02\times10^{34} J.s\)
In this option, the given value is in three significant figures but this value is not the Planck constant value.
So, this is the incorrect option.
Hence, the correct exponential notation for Planck's constant is\(6.63\times10^{-34} Js\).
The correct option is (b).
Reference :
https://brainly.com/question/15939529
The ability to conduct an electric current, dissolve in water (solubility) , and melt are _______________.
Group of answer choices
chemical bonding
physical properties
physical changes
chemical properties
Answers
Answer:
chemical bonding
Explanation:
i hope this helped :)
28) Correctly record
the volume of this
box.

Answers
Answer:
1653.21 cm
Explanation:
volume formula of rectangular prism is volume times height times width
40.1 times 4.457 times 9.25 = 1653.21
Answer:
The volume of cuboid is 1653.21 cm³
Step-by-step explanation:
GIVEN :
Length of cuboid = 40.1 cmBreadth of cuboid = 4.457 cmHeight of cuboid = 9.25 cmTO FIND :
Volume of cuboidUSING FORMULA :
\(\quad{\star{\underline{\boxed{\sf{V_{(cuboid)} = lbh}}}}}\)
V = volume l = length b = breadth h = heightSOLUTION :
Substituting all the given values in the formula to find the volume of cuboid :
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = lbh}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = l \times b \times h}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 40.1 \times 4.457 \times 9.25}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 178.7275 \times 9.25}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 1653.21272}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} \approx 1653.21}}}\)
\(\quad{\star{\underline{\boxed{\sf{V_{(cuboid)} = 1653.21 \: {cm}^{3}}}}}}\)
Hence, the volume of cuboid is 1653.21 cm³.
————————————————What do the graphs and images in the reference image represent? Explain their correlation.

Answers
Fill in the table with the mass of each sample. Use the periodic table to find the molar mass of each compound. Then calculate moles and particles. Periodic TableThe two compounds have the same number of moles. Compare how the number of moles relates to the masses and number of particles for each compound.

Answers
1) First, let's calculate the molar mass of NaCl and CoCl₂:
Atomic mass of:
Na - 22.99 g/mol
Cl - 35.45 g/mol
Co - 58.93 g/mol
So for NaCl:
22.99 + 35.45 = 58.44 g/mol
Molar mass of NaCl = 58.44 g/mol
For CoCl₂:
58.93 + (2x35.45) = 129.83 g/mol
Molar mass of CoCl₂: 129.83 g/mol
2) Now let's find out the number of moles of each sample. For this, we use the following equation:
moles = mass / molar mass
NaCl:
mole = 116.8 / 58.44
mole = 1.999 moles of NaCl
CoCl₂:
mole = 259.7 / 129.83
mole = 2.000 moles of CoCl₂
3) To find the amount of particles, we use the Avogadro's constant. Avogadro's constant is a constant of proportion between the amount of matter and the number of entities that are linked to that amount. These entities can be atoms, molecules, ions, electrons, protons, neutrons.
In this case, we have the same number of moles, so we will have the same number of particles for both NaCl and CoCl₂.
someone pls answer i will in fact give u brainliest

Answers
Answer:
d. Solid
Explanation:
It is frozen to be a glacier
Hope this helps :)
halp balance plez (⊙◡⊙)

Answers
Answer: SnO2 + 2 H2 = Sn + 2 H2O
Explanation: I used a balance equation website. It's called WebQC if you want to check it out for future help.
2. How many moles are in 7.30 X 10^23 molecules of NaCl?
Answers
Answer:
\( \huge{ \boxed{1.213 \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L}} \\ \)
where
n is the number of molesN is the number of entitiesL is the Avogadro's constant which is 6.02 × 10²³ entitiesFrom the question
N = 7.30 × 10²³ NaCl molecules
\(n = \frac{7.30 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{7.30}{6.02} \\ = 1.2126\)
We have the final answer as
1.213 moles200 L of a gas at 10 atm pressure and 400 K is cooled to 100 K and reduced to 2 atm pressure. What is the new volume?
Answers
Answer:
250L
Explanation:
Data;
V1 = 200L
P1 = 10atm
T1 = 400K
T2 = 100K
P2 = 2atm
V2 = ?
To solve this question, we'll have to use the combined gas equation which is a combination of Boyle's law, Charles law and pressure law.
From combined gas equation,
(P1 × V1) / T1 = (P2 × V2) / T2
P1 × V1 × T2 = P2 × V2 × T1
Solve for V2,
V2 = (P1 × V1 × T2) / P2 × T1
V2 = (10 × 200 × 100) / (2 × 400)
V2 = 200,000 / 800
V2 = 250L
The final volume of the gas is 250L
A 35.00 gram sample of a substance containing only Carbon, Hydrogen and Oxygen was combusted. The products of this combustion reaction were shown to be 51.29 grams of CO2 and 20.99 grams of H2O. Determine the empirical formula of the original compound given this combustion analysis data.
Answers
The empirical formula of the original compound given the combustion analysis data is CH₂O
How to determine the mass of Carbon Mass of CO₂ = 51.29 gMolar mass of CO₂ = 44 g/mol Molar of C = 12 g/mol Mass of C =?Mass of C = (12 / 44) × 51.29
Mass of C = 13.99 g
How to determine the mass of HMass of H₂O = 20.99 gMolar mass of H₂O = 18 g/mol Molar of H = 2 × 1 = 2 g/mol Mass of H =?Mass of H = (2 / 18) × 20.99
Mass of H = 2.33 g
How to determine the mass of OMass of compound = 35 gMass of C = 13.99 gMass of H = 2.33 gMass of O =?Mass of O = (mass of compound) – (mass of C + mass of H)
Mass of O = 35 – (13.99 + 2.33)
Mass of O = 18.68 g
How to determine the empirical formula C = 13.99 gH = 2.33 gO = 18.68 gEmpirical formula =?Divide by their molar mass
C = 13.99 / 12 = 1.1658
H = 2.33 / 1 = 2.33
O = 18.68 / 16 = 1.1675
Divide by the smallest
C = 1.1658 / 1.1658 = 1
H = 2.33 / 1.1658 = 2
O = 1.1675 / 1.1658 = 1
Thus, the empirical formula of the compound is CH₂O
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1