part 5 out of 5 the 1° alkyl halide is also allylic, so it forms a resonance-stabilized carbocation. increasing the stability of the carbocation by resonance (select) the rate of the sn1 reaction.
Answers
The increased stability of the carbocation due to resonance stabilization will result in an enhanced rate of the SN1 reaction.
How to find the effect of resonance stabilization?In the given context, when a 1° alkyl halide is also allylic, it forms a resonance-stabilized carbocation due to the presence of adjacent double bonds. The resonance stabilization of the carbocation increases its stability.
In an SN1 reaction (substitution nucleophilic unimolecular), the rate-determining step involves the formation of the carbocation intermediate. A more stable carbocation will have a lower energy barrier for its formation, leading to a faster rate of the SN1 reaction.
Therefore, the increased stability of the carbocation due to resonance stabilization will result in an enhanced rate of the SN1 reaction.
Learn more about resonance stabilization
brainly.com/question/31040880
#SPJ11
Related Questions
Objects with potential energy got that energy:
A. Entirely from their height above the earth.
B. After work was performed on them.
C. When kinetic energy was released from fossil fuels.
D. Through electromagnetic discharges.
Answers
Answer:
D. Through electromagnetic discharges.
Explanation:
when temperature increase gas decrease or increase support u r answer with daily life example
Answers
Answer:
increase
Explanation:
dont known reason burr answer is correct
Answer:
When temperature increases ,gas increases ,because of the rise in temperature evaporation will take place.
Explanation:
When you boil water to make coffee/tea the kettle/boiler releases the evaporation caused by the rise in temperature.
Which is the correct order of orbital filling according to the aufbau principle?a 4s 4p 4d 4f b 4p 4d 5s 4f c 4s 3d 4p 5s d 4d 4f 5s 5p
Answers
The correct order of orbital filling according to Aufbau principle is
(a) 4s 4p 4d
According to the Aufbau principle, electrons must occupy all of an atom's accessible orbitals, working their way up from the lowest energy level to the highest, to ensure that both the electrons and the atom have the least amount of energy possible and are stable.
As a result, electrons begin to occupy orbits in increasing order of "n" value, starting with the first orbit (n = 1).
If any orbital's primary quantum number is "n,"
n2 is the number of orbitals, and 2n2 is the number of electrons in any n-shell.
The orbital's's' sub-orbital count is 1′. P is three, while D is five.
There can be a total of two electrons in each orbital 0f an atom
To learn more on filling of orbitals please refer:
https://brainly.com/question/11316046
#SPJ4
The correct order to fill the orbitals according to the Aufbau's principle is:
(a) 4s 4p 4d
According to the Aufbau principle, electrons occupy all accessible orbitals of an atom, rising from the lowest energy level to the highest energy level so that both the electron and the atom are at the lowest possible energy and are stable you need to make sure that
As a result, electrons start occupying orbitals in order of increasing "n" value, starting with the first orbital (n=1).
If the first quantum number of the orbital is "n",
n2 is the number of orbitals and 2n2 is the number of electrons in each n shell.
The number of s orbitals in the orbital is 1'. P is 3 and D is 5. There can be a total of two electrons in each orbital of an atom.
To Read more about orbital filling please refer:
Brainly.com/question/11316046
#SPJ4
START
What is the
mass of 2.75
moles of CO₂?
Answers
The mass of 2.75 moles of CO2 is approximately 121.03 grams.
What is the mass of 2.75 moles of CO₂?To calculate the mass of 2.75 moles of CO2, we need to use the molar mass of CO2.
The molar mass of CO2 is approximately 44.01 g/mol (12.01 g/mol for carbon + 2x 16.00 g/mol for oxygen).
To calculate the mass of 2.75 moles of CO2, we can use the following formula:
mass = moles x molar mass
Substituting the given values, we get:
mass = 2.75 moles x 44.01 g/mol
mass = 121.0275 g
Learn more about molar mass here: https://brainly.com/question/837939
#SPJ1
What is the electron configuration for Selenium? *
1s²2s²2p⁶3s²3d¹⁰3p⁶4s³4p³
2s³2p⁶3s³3p⁶4s³3d¹⁰4p⁶
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁴
1s²2s³2p⁶3s²3p⁶3d¹⁰4s²4p⁴
Answers
Explanation:
The electronic configuration of Selenium(Se) is 1s22s22p63s23p63d104s24p4 we remove electron .the electron configuration is1s22s22p63s23p63d104s24p4
the correct anwser is C
The electron configuration for Selenium is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁴.
Therefore, option c is correct.
What is electronic configuration ?The electronic configuration of an element is the filling of electron from lower energy level to the higher energy level. This is according to the Aufbau's principle.
Selenium has 34 electrons. The electronic configuration of Selenium can be determined by filling the electrons in increasing order of energy levels and sub-levels.
The electronic configuration of Selenium can be written as:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁴
Here, 4s is more energetic than 3d and thus occupies first.
Find more on electronic configuration:
https://brainly.com/question/29757010
#SPJ7
what is the number of shells and number of valence electrons for the following elements: Na, O, I, Ca
Answers
The number of electrons present in the outermost shell is called valence electrons.
What are valence electrons?The number of electrons present in the last shell is called valence electrons. The atomic number of sodium is 11 and the atomic number of oxygen is 8.
There is 1 valence electron in sodium. The number of valence electrons in oxygen is 2.
The atomic number of iodine is 53. The number of the valence electron in iodine is 1. The atomic number of calcium is 20. The valence electron in calcium is 2.
Therefore, The number of electrons present in the outermost shell is called valence electrons.
To know more about valency, refer to the link:
https://brainly.com/question/12744547
#SPJ1
Photochemical smog results from the interaction of pollutants in the presence of.
Answers
Photochemical smog results from the interaction of pollutants in the presence of sunlight.
Photochemical smog is a type of air pollution that forms when **pollutants** such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of **sunlight**. This process occurs in the lower atmosphere and can lead to the formation of harmful substances like ozone and particulate matter. Major sources of these pollutants include vehicle emissions, industrial processes, and the use of solvents. The formation of photochemical smog is highly dependent on weather conditions, with increased temperatures and sunlight contributing to more severe smog events. Reducing emissions of pollutants and promoting cleaner technologies can help mitigate the formation of photochemical smog and its impacts on human health and the environment.
Know more about photochemical smog here:
https://brainly.com/question/27960448
#SPJ11
45) What is the limiting reactant for the following reaction given we have 2.6 moles of HCl and 1.4 moles of Ca(OH)2?
Reaction: 2HCl + Ca(OH)2 → 2H2O + CaCl2
A) Ca(OH)2
B) HCl
C) H2O
D) CaCl2
E) not enough information
Answers
We can use the mole ratios of HCl and \(Ca(OH)$_2$\) to calculate the amount of product produced by each, and identify the limiting reactant.Option (A)
From the balanced chemical equation, we can see that 1 mole of \(Ca(OH)$_2$\) reacts with 2 moles of HCl to produce 1 mole of\(CaCl$_2$\)and 2 moles of \(H$_2$O\). Therefore, we can use the mole ratios to calculate the amount of product that can be produced from the given amount of each reactant:
For 2.6 moles of HCl:
\(2.6,mol,HCl \times \frac{1,mol,Ca(OH)_2}{2,mol,HCl} = 1.3,mol,Ca(OH)_2\)
For 1.4 moles of :\(Ca(OH)$_2$\)
\(1.4,mol,Ca(OH)_2 \times \frac{2,mol,HCl}{1,mol,Ca(OH)_2} = 2.8,mol,HCl\)
We can see that 1.3 moles of , \(Ca(OH)$_2$\)can react with 2.6 moles of HCl to produce 2.6 moles of \(H$_2$O\) and 1.3 moles of \(CaCl$_2$\) Therefore, \(Ca(OH)$_2$\)is the limiting reactant.
The answer is: \(\boxed{\text{(A) Ca(OH)2}}$.\)
Learn more about chemical equation
https://brainly.com/question/30087623
#SPJ4
Adding or removing thermal energy causes a change in state of matter.
O True
O False
Answers
Answer:"You can change an object's state of matter by adding or removing thermal energy. When you add thermal energy to an object, these things can happen: Particles move faster (increased kinetic energy). Particles get farther apart (increased potential energy)."
Explanation:
Which is the amount of matter in an object?
Answers
Answer:
Mass
Explanation:
(not sure if this is what you were looking for, but mass is the amount of matter in an object)
hich of the following substances would not exhibit any hydrogen bonding interactions in a pure substance
Answers
N(CH₃)₃ will not exhibit hydrogen bonding.
What is hydrogen bonding?
The term "hydrogen bond" refers to the electrostatic force of attraction between a hydrogen atom in one polar molecule (such as water) and a tiny electronegative atom (such as oxygen, nitrogen, or fluorine) in typically another molecule of the same or different polar substance.
When a hydrogen atom forms a covalent connection with the extremely electronegative N, O, or F atoms, it produces a significant partial positive charge on the H atom. N(CH₃)₃ would not display hydrogen bonding because it is the only alternative available without H directly bonded to N, O, or F (in this case, there are only C-H bonds).
I understand the question you are looking for is this:
Which of the following substances would not exhibit any hydrogen bonding interactions in a pure substance?
A) H₂O
B) HF
C) N(CH₃)₃
D) CH₃CH₂OH
E) CH₃NH₂
Learn more about hydrogen bonding here:
https://brainly.com/question/12408823
#SPJ4
If the atomic mass unit (AMU) is the average mass of all the isotopes of an element; what is the mass number of an element?
Answers
Answer:
If this help please set brainly brainliest.
The mass number of an element is the sum of the number of protons and neutrons in the nucleus of an atom of that element. It is typically represented by the symbol A. For example, the mass number of carbon-12, which is one of the stable isotopes of carbon, is 12 because it has 6 protons and 6 neutrons in its nucleus. The atomic mass unit (AMU) is a unit of mass that is used to express the masses of atoms and molecules. It is defined as 1/12 the mass of a carbon-12 atom, which is approximately 1.66 x 10^-27 kilograms. Isotopes are atoms of the same element that have the same number of protons in their nuclei but a different number of neutrons. Because they have the same number of protons, they have the same atomic number and are therefore the same element. However, because they have a different number of neutrons, they have a different mass number and therefore a slightly different atomic mass. The atomic mass of an element is usually given as the average mass of all of its isotopes, taking into account their natural abundances.
when two atomic nuclei come together to form a new species of atom, it is called:
Answers
Answer:
nuclear fusion
Explanation:
When two atomic nuclei come together to form a new species of atom, the process is called nuclear fusion. In nuclear fusion, the nuclei of two lighter elements combine to create a heavier element, typically releasing energy in the process.
This reaction occurs under extremely high temperatures and pressures, allowing the positively charged nuclei to overcome the electrostatic repulsion between them and get close enough for the strong nuclear force to bind them together.
One common example of nuclear fusion is the fusion of hydrogen nuclei to form helium in the core of stars, including our Sun. This process, known as the proton-proton chain, powers the Sun's energy output and is essential for maintaining the stable conditions necessary for life on Earth. Nuclear fusion reactions also release vast amounts of energy in the form of light and heat, which is why stars emit such intense radiation.
Scientists have been working for decades to develop nuclear fusion as a clean and practically limitless energy source on Earth. If harnessed successfully, fusion energy could significantly reduce our dependence on fossil fuels and help address the global energy crisis. However, achieving the necessary conditions for controlled fusion reactions has proven to be challenging, and practical fusion power generation remains an ongoing area of research and development.
Learn more about fossil fuels here:
https://brainly.com/question/3371055
#SPJ11
what is considered cisgender?
Answers
Answer:
that means whatever gender you are now is the same as what was presumed for you at birth.
3.0 Liters of gas at a pressure of 0.90 atm is compressed to 2.0 atm.
What is the new volume?
Answers
Answer:
5.5 L
Explanation:
Which set of conditions are correct
for the reaction below?
CH4 + O2 → CO + H₂O
A. complete combustion & not balanced
B. incomplete combustion & not balanced
C. incomplete combustion & balanced
D. complete combustion & balanced
Answers
Answer: D. Complete Combustion & Balanced
Explanation:
D. Complete Combustion & Balanced
Complete combustion is a type of combustion reaction that occurs when a fuel reacts with oxygen to produce carbon dioxide and water as products. In a complete combustion reaction, the fuel is burned completely and all of the reactants in the reaction are used up.
A balanced reaction means that the number of atoms of each element on the reactant side of the equation is equal to the number of atoms of each element on the product side of the equation. The reaction shown above is balanced, meaning that there are 4 atoms of carbon and 8 atoms of oxygen on the reactant side, and 1 atom of carbon and 2 atoms of oxygen on the product side.
Answer:
D. complete combustion & balanced
A sample of gas has a volume of 5.0 L and a pressure of 2.92 atm. If the final volume is 5.7 L, what is the final pressure of the gas?
Show your work in the work space below.
Hint: Use the equation for Boyle's Law
Answers
(2.92)(5)=P2(5.7)
14.6=P2(5.7)
P2=2.56atm
What pressure is exerted by 0.375 mol of hydrogen gas in a 4.33 L container at 55.0C?
Answers
Answer:
47
Explanation:
9
23 What is a use of sulfuric acid?
A
as a bleach
B.
as a food preservative
in the manufacture of detergents
D.
in the manufacture of
vanadium(V) oxide, V205
Answers
Answer:
B. as a food preservative in the manufacture of detergents
.........
The temperature of a sample of liquid water changes from 50°C to 30°C. Which statement best explains the change
that must happen at the molecular level?
The molecules move more quickly and their average kinetic energy increases
The molecules move more bowly and their average kinetic energy decreases
The molecules move more quickly and their average kinetic energy decreases
The molecules move more slowly and their average kinetic energy increases.
Answers
Answer:
The molecules move slower than the temp of 50°c and their average kinetic energy decreases.
Explanation:
kinetic energy cannot increase as temperature is reduced. molecules will still move with reduced motion.
pls help me with this
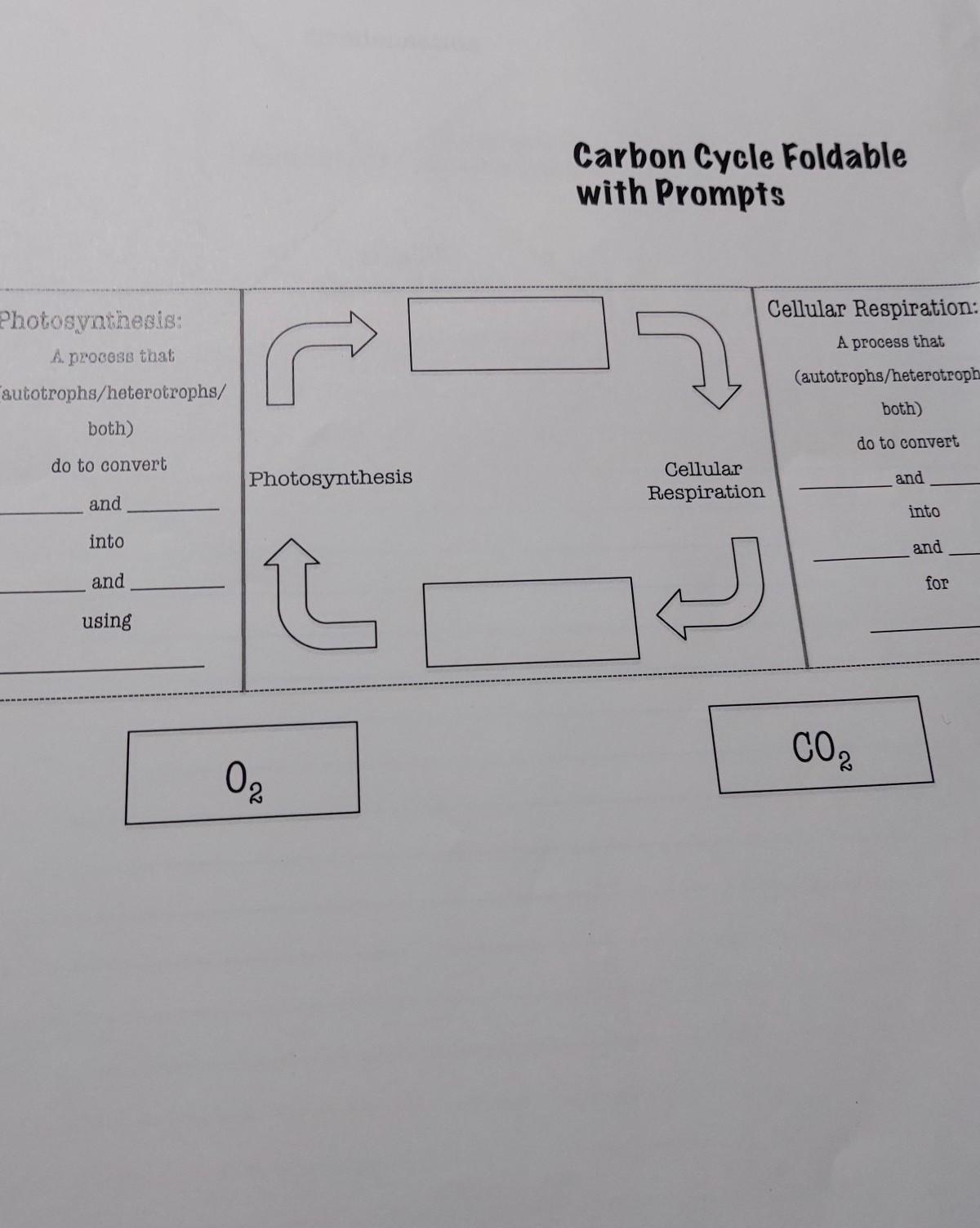
Answers
Where would you expect light waves to move fastest?
A. Through an iceberg
B. On a mountaintop
C. In space
D. Under the sea
Answers
In space light waves move fastest, which is C. This is due to the fact that space is completely open and unrestricted and contains nothing that would do so.
What is Light waves?
The region of the electromagnetic spectrum that the human eye perceives as light, or visible light, is made up of electromagnetic radiation. Typically, visible light is characterised as having wavelengths between 400 and 700 nanometers (nm), or frequencies between 750 and 420 terahertz, which fall between the longer-wavelength infrared and the shorter-wavelength ultraviolet (with shorter wavelengths). The term "light" in physics can be used to more broadly describe electromagnetic radiation of any wavelength, whether or not it is visible. Gamma rays, X-rays, microwaves, and radio waves are all forms of light in this sense. Intensity, propagation direction, frequency or wavelength spectrum, and polarisation are the four main characteristics of light.
To learn more Light waves
https://brainly.com/question/15033563
#SPJ9
draw complete lewis dot structures for ethyl acetate, acetaminophen, phenacetin, caffeine, and aspirin. after analysis of the lewis structure of ethyl acetate (which is used as the mobile phase in this experiment), determine its polar or nonpolar nature. using the structure of ethyl acetate, is it a polar or a non-polar solvent?
Answers
The Lewis structure is a simplified representation of the valence shell electrons in a molecule. It is used to show how electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or to connect electrons as a line between two atoms.
1) Ethyl acetate: \(C_{4}H_{8}O_{2}\)
Ethyl acetate is a widely used solvent, especially for paints, varnishes, lacquers, cleaning compounds, and perfumes. Like last week's MOTW, dichloromethane, is used as a solvent to reduce coffee grounds. In the laboratory, ethyl acetate is a common solvent for column and thin layer chromatography.
2. Acetaminophen:
Acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription medications. It relieves pain and fever. Acetaminophen can also be combined with other active ingredients in medications that treat allergies, coughs, colds, flu, and insomnia. In prescription acetaminophen is found along with other active ingredients to treat severe pain. Acetaminophen can cause serious liver damage if used more than prescribed. The FDA has taken action to improve consumer safety when using acetaminophen.
3) Phenacetin:
Phenacetin has been used as an analgesic and antipyretic in both human and veterinary medicine for many years. It was introduced in treatment in 1887 and was widely used in analgesic mixtures until it was included in kidney diseases (nephropathy) due to the abuse of analgesics. Phenacetin was also once used as a stabilizer for hydrogen peroxide in hair bleaching preparations.
4-Caffeine
Caffeine is a stimulant, which means it increases activity in your brain and nervous system. Caffeine also increases the circulation of chemicals in the body like cortisol and adrenaline. In small doses, caffeine can make you feel relaxed and focused.
5.Aspirin
Aspirin is a common medicine to relieve minor aches, pains, and fever. Also, people use it as an anti-inflammatory or blood thinner.
Aspirin can buy by people can buy over the counter without a prescription. Daily benefits include relieving headaches, reducing inflammation, and reducing fever.
For more such question on Lewis Dot Structure:
https://brainly.com/question/20300458
#SPJ4



Which of the following statements about groundwater are TRUE? Select ALL that apply. (Hint: 4 of the 6 statements are correct!) The water table generally maintains a relatively flat shape beneath the ground surface. Owing to pressure differences, it is possible for groundwater to flow uphill. The water table will be deeper below the surface in arid regions as compared to humid regions. A confined aquifer is an underground source of water that is prevented from reaching Earth's surface by an impermeable layer. The surface of the water table may be visible above ground as the surface of a river or lake. If an aquifer has a high porosity, then it will also have a high permeability
Answers
When opposed to humid areas, ground water will be deeper in arid areas. underground water source termed an confined aquifer is one in which an impermeable layer blocks the water's path to the surface the planet.
What places can one find constrained aquifers?An aquifer that has restricting beds enclosing it on both the top and bottom is said to be confined. At substantial depths below the surface of the ground, confined aquifers typically exist.
What source of water do restricted aquifers use?Aquifers that are enclosed and filled with water are those that are found beneath the surface of the land. The aquifer is under pressure because of the impermeable layers that are both before and below it. As a result, when a well drills into the aquifer, water will rise over the top of the impermeable layers.
To know more about confined aquifers visit:
https://brainly.com/question/14916917
#SPJ4
2. if you or one of your laboratory partners misread the procedure and added 100 ml of distilled water to the solid khp rather than 50 ml of water (procedure 1.5), how would this affect the concentration of sodium hydroxide solution that you measured? explain briefly. (10 points)
Answers
If 100 ml of distilled water was added to the solid khp instead of 50 ml (as stated in procedure 1.5), the concentration of the sodium hydroxide solution that was measured would be lower than the actual concentration.
The concentration of the sodium hydroxide solution is determined by titrating it with the khp solution. The khp solution is prepared by dissolving solid khp in water, and the concentration of the khp solution is dependent on the amount of water added to the solid khp. If more water than required is added, the resulting khp solution would be more dilute, which would lead to a lower concentration of the sodium hydroxide solution when titrated.
Therefore, adding 100 ml of distilled water instead of 50 ml to the solid khp would dilute the khp solution, resulting in a lower concentration of the sodium hydroxide solution that was measured during titration. It is important to carefully follow the procedures to obtain accurate results in the laboratory.
To know more about titration, visit:
https://brainly.com/question/29590776
#SPJ11
which is a better conductor of eclectic current: a solid ionic compound or a melted ionic compound
Answers
Answer:
Melted ionic compounds
Explanation:
This is because the ions have dissociated and for electricity to pass through a substance it is important that the substance must have ions present in it.
Which factor plays the biggest role in delaying the detection of childhood
diseases?
Answers
Answer:
poor access to health care providers
Explanation:
without health care providers you cant get tested.
What element has the electron configuration below? *
please help me

Answers
Answer:
Symbol Ar
Group 18
Electron configuration- 1s² 2s² 3p6 3s² 3p6
Explanation:
The 6 is small and will be placed in top but I don't have the option that's why I wrote like that
which of these changes are endothermic and which are exothermic
baking a cake
bending a plastic ruler
cutting paper
melting ice cream
ripening of fruit
souring of milk
Answers
Answer:
exothermic - bending a plastic ruler , cutting paper, melting ice cream, ripening of fruit, souring of milk.
Explanation:
endothermic- baking of cake .
Consider the reaction
4Fe + 30, - 2FeO,
Round to 2 decimals for your response. If 540g of iron react with 400g of oxygen gas, which will be the limiting reactant? 540g of
iron can produce
type your answer... moles of iron oxide. And, 400g of oxygen gas can produce type your answer...
moles of iron oxide. choose your answer...
will be the limiting reactant.

Answers
For Iron :
n Fe = m Fe / Mr Fe
n Fe = 540 / 55.85
n Fe = 9.67 mol
n Fe₂O₃ = (2/4) • 9.67
n Fe₂O₃ = 4.83 mol
For Oxygen :
n O₂ = m O₂ / Mr O₂
n O₂ = 400 / 32
n O₂ = 12.5 mol
n Fe₂O₃ = (2/3) • 12.5
n Fe₂O₃ = 8.33 mol
Fe is the limiting reactant