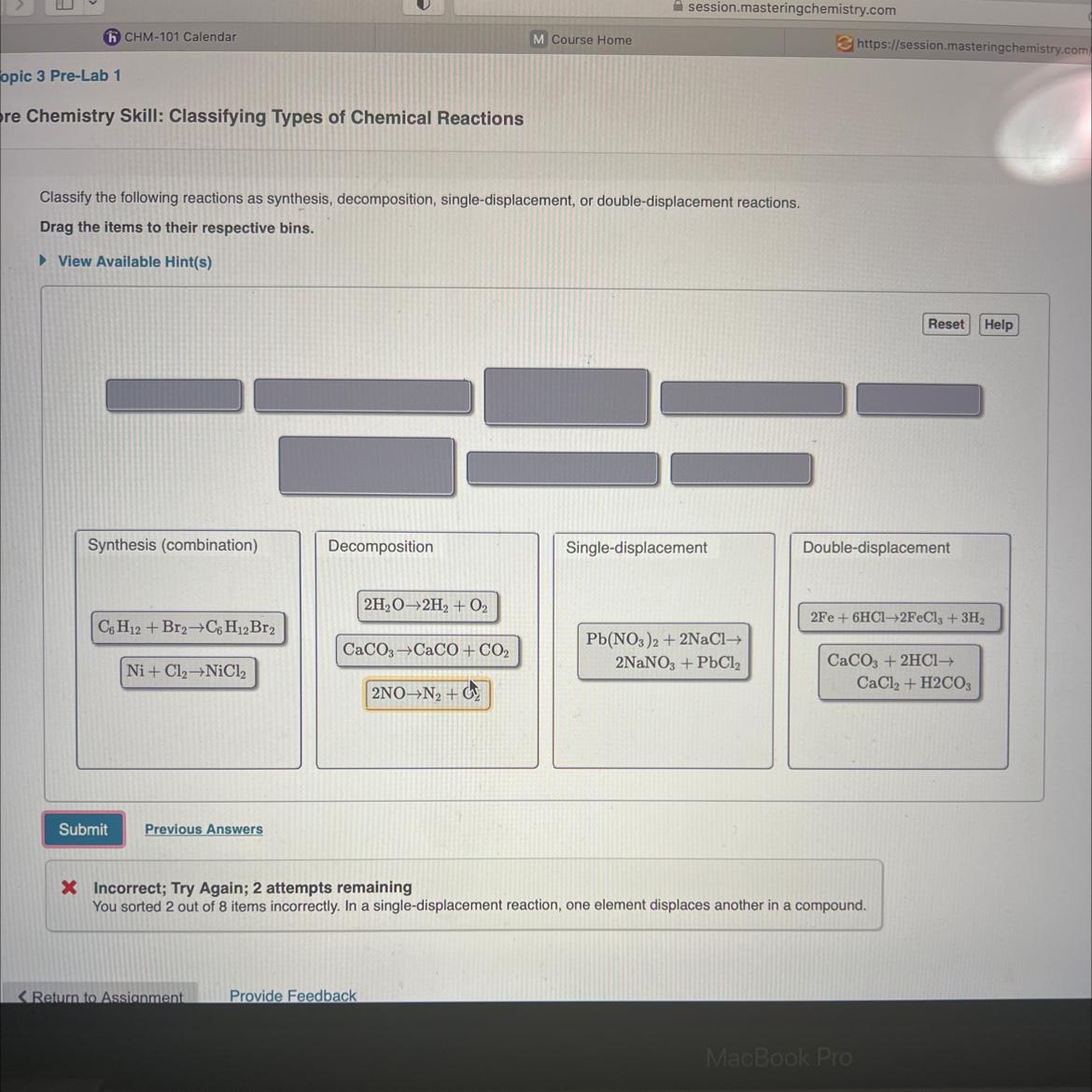
Please help! I have 2 remaining attempts and only 2 of my answers are incorrect. Please let me know which reactions are wrong and where they should be put! LOTS OF POINTS!!!!
Answers
I think the second one needs to be put on the sixth area.
Related Questions
the combustion of ammonia in the prescne of excess oxygen yield no2 and h2o. how many grams of no2 are produced when 43.9mg of ammonia burn
Answers
The combustion of ammonia in the prescne of excess oxygen yield no2 and h2o. 0.119 grams of no2 are produced when 43.9mg of ammonia burn.
Molar mass of NH3 = 1*MM(N) + 3*MM(H)
= 1*14.01 + 3*1.008
= 17.034 g/mol
mass of NH3 = 0.0439 g
mol of NH3 = (mass)/(molar mass)
= 0.0439/17.034
= 2.577*10^-3 mol
From balanced chemical reaction, we see that
when 4 mol of NH3 reacts, 4 mol of NO2 is formed
mol of NO2 formed = moles of NH3
= 2.577*10^-3 mol
Molar mass of NO2 = 1*MM(N) + 2*MM(O)
= 1*14.01 + 2*16.0
= 46.01 g/mol
mass of NO2 = number of mol * molar mass
= 2.577*10^-3*46.01
= 0.119 g
To learn more about Molar mass visit:https://brainly.com/question/22997914
#SPJ4
please help
Which of the following diagrams shows how osmosis would affect a plant cell when placed in a solution with a lower solute concentration than its own?
A.
Diagram Y
B.
Osmosis is not dependent on solute concentration.
C.
Diagram Z
D.
Diagram X

Answers
Answer:
Diagram Z
Explanation:
A cell placed into a hypotonic solution will swell and expand until it eventually burst through a process known as cytolysis.
Answer:
Diagram Y
Explanation:
Osmosis is the passive movement of water molecules across (into or out of) a cell membrane. "Passive" means that the process occurs automatically without the need for chemical energy.
In the diagrams above, a plant cell is shown as it would appear in a solution with a solute concentration more than its own (X), a solution with a solute concentration equal to its own (Y), and a solution with a solute concentration less than it own (Z). The movement of water is toward the area of more solute concentration (and therefore less water concentration).
The answer, then, is Diagram Y.
(Trust me, I just did it)
How is the process of using patterns to predict phases of the moon similar to the process Mendeleev used to predict undiscovered elements?
Answers
The characteristic of some repeating property can be used to find the answer is
The periodic table and the moon phases can be ordered according to some repeating property, you can create an ordered table and leave the gaps for the unknown properties.
The periodic table created by Mendeleev was initially based on the atomic weight of the elements, but he realized that the similarities of the atoms were greater if he used the atomic number, so he changed the ordering.
Mendeleev found that elements with the same number of electrons in their last shell had similar properties, when he ordered them he found that there were holes in the table, shortly after the work of many researchers the holes were completed and found numerous new chemical elements.
In the observation of the phases of the Moon around the earth it is found that for short measurements there are four phases, for longer measurements in addition to these four phases there are several higher repetition periods, with repeated observation the phases and periods are found. .
We can see that the two jobs are searched for, which is repeated and a table is created, leaving the spaces that are not known empty, with subsequent jobs filling these holes.
In conclusion, with the characteristics of some repeating property, the answer can be found is
The periodic table and the phases of the moon can be ordered according to some repeating property, you can create an ordered table and leave the gaps for the unknown properties.
Learn more about the periodic table here:
https://brainly.com/question/3376367
In the nitrate reduction test, what would the result be after solutions a and b were added to a culture that had reduced nitrate to ammonia?.
Answers
In the nitrate reduction test , The result would be after solution a and b were added to a culture that had reduced nitrate to ammonia that no color would form.
The nitrite reduction based on the measurement of nitrite. when we add zinc to the reduction of nitrate to nitrite and if the the red color developed after addition of zinc then it means nitrate was not reduce. if there is no color obtain after adding the zinc then nitrate was completely reduced to nitrite.
Thus, In the nitrate reduction test , The result would be after solution a and b were added to a culture that had reduced nitrate to ammonia that no color would form.
To learn more about nitrate reduction test here
https://brainly.com/question/20308712
#SPJ4
why would scientists use a spectrometer in space.
A. To study the chemical composition of the surface of a planet
B. To detect the birth and death of distant start and galaxies
C. To convert light from stars into usable energy for humans
D. To maintain a constant air supply on board a space shuttle
Answers
Answer:
A. To study the chemical composition of the surface of a planet
Explanation:
Strictly speaking, a spectrometer is any instrument used to view and analyze a range (or a spectrum) of a given characteristic for a substance (for example, a range of mass-to-charge values as in mass spectrometry), or a range of wavelengths as in absorption spectrometry like nuclear magnetic radiation spectroscopy
-hope this helps!
Explain which of the two, triethylammonium chloride ((ch3ch2)3n-hcl) or triethylamine ((ch3ch2)3n) is more soluble in water and why
Answers
Triethylamine ((CH3CH2)3N) is less soluble in water than ((CH3CH2)3N•HCl). Compared to the uncharged N atom in triethylamine, the positive charge on the N atom in triethylammonium chloride is more polar.
What is triethylammonium chloride?If your compound is not susceptible to moisture, you should dissolve it in dichloromethane or chloroform before giving it a brief wash in cold water and separating it. Cold water washing reduces the product's water solubility. Triethylamine and HCl (strong acid) are combined to form the salt triethylammonium chloride (weak base).
Chloroform readily dissolves the salt of triethylamine hydrochloride. To produce 93 grammes of crude using triethylamine hydrochloride salt, chloroform is first removed. In order to get a certain acidic pH, it is dissolved in 250 mL of water, cooled to 10 °C, then acidified by adding 10% hydrochloric acid.
Triethylamine ((CH3CH2)3N) is less soluble in water than ((CH3CH2)3N•HCl). Compared to the uncharged N atom in triethylamine, the positive charge on the N atom in triethylammonium chloride is more polar. Enhanced solubility results from greater interactions with water as a result of this increased polarity.
To learn more about triethylammonium chloride refer to:
https://brainly.com/question/18521023
#SPJ4
What are the condiction requried for
work to be done
Answers
Answer:
the object should be displaced on which the force is applied
Explanation:
HOPE IT HELPS YOU
A cube of gold weighing 185g is heated from 20.0°C to some higher temperature, with the absorption of 225 joules of heat. The specific heat of gold is 0.030 J/g°C. What was the final temperature of the gold?
Answers
A cube of gold weighing 185g is heated from 20.0°C to some higher temperature, with the absorption of 225 joules of heat. The specific heat of gold is 0.030 J/g°C. 60.5°C was the final temperature of the gold.
What is gold?Gold has the chemical symbol Au (from Latin: aurum) as well as the atomic number 79. As a result, it is one among the highest atomic number elements found in nature.
In its pure state, it is a brilliant, somewhat orange-yellow, dense, soft, malleable, extremely ductile metal. Gold is indeed a transition metal as well as a group 11 element chemically.
225 = 185 × 0.030×(final temperature- 20.0)
225 =5.55×(final temperature- 20.0)
225/ 5.55 = final temperature- 20.0
60.5 = final temperature
Therefore, 60.5°C was the final temperature of the gold.
To know more about gold, here:
https://brainly.com/question/24004315
#SPJ1
In North America, over \( 90 \% \) of the grain grown is used to feed livestock. True False Question 42 2 pts What are the two types of erosion? physical erosion and chemical erosion chemical erosion
Answers
The statement "In North America, over 90% of the grain grown is used to feed livestock" is TRUE. Regarding the question "What are the two types of erosion?", the answer is physical erosion and chemical erosion.
Erosion is the process of wearing or grinding something down, often by water, wind, or ice. Erosion occurs when the Earth's surface is disturbed or exposed to certain elements.
It can be defined as the natural process of carrying away or removing soil, rock, or other materials from the Earth's surface by water, wind, or other natural forces.Physical erosionPhysical erosion is caused by natural processes such as wind, water, or ice, and it occurs when rocks and soil are loosened and removed by a natural agent.
Physical erosion may be caused by things such as earthquakes, volcanoes, glaciers, and landslides.Chemical erosionChemical erosion is caused by chemical processes such as acid rain and the breakdown of rocks by chemical reactions. Chemical erosion can be caused by things such as rainwater, acid rain, and groundwater that dissolves minerals and rocks.
To know more about erosion visit:
https://brainly.com/question/30587260
#SPJ11
A 5.0 mole sample of gas has 54 mmHg of pressure at 273K. What is the volume of the gas?
(Round your answer to the correct number of significant figures)
Answers
Answer:
here's the answer hope it helps

What is the percent composition of a compound formed when 6.85 g of magnesium combines with 20.0 g of chlorine to form magnesium chloride?
Answers
Answer:
Mass of Magnesium Chloride = 26.85 grams
Percentage of Magnesium= 25.5 %
Percentage of Chlorine =74.5 %
Explanation:
Mass of Magnesium Chloride= Mass of magnesium + Mass of Chlorine 6.85 grams + 20.0 grams = 26.85 grams
Percentage of Magnesium = 6.85 grams/26.85 grams x 100% = 25.5%
Hence, Magnesium = 25.5 %
Percentage of Chlorine = (20.0 grams/26.85 grams) x 100 % = 74.5%
Hence, Chlorine = 74.5 %
So, the compound is 25.5 % magnesium and 74.5 % Chlorine by mass.
Which of the following reactions of alkenes is NOT stereospecific? A Hydrogenation (H2/P1) o B Bromination (Br2 in CH2Cl2) o c Acid-catalyzed hydration (H20/H2504) O D Bromohydrin formation (Br2/H20)
Answers
The following reactions of alkenes is not stereospecific is C. acid-catalyzed hydration reaction (H₂O/H₂SO₄)
Stereospecific reactions occur when the stereochemistry of the reactant is retained in the product. These types of reactions are distinguished by the use of double-headed arrows in reaction mechanisms to demonstrate the conservation of stereochemistry. The following reactions of alkenes stereospecific are hydrogenation (H₂/P₁), bromination (Br₂ in CH₂Cl₂), bromohydrin formation (Br₂/H₂O).
The acid-catalyzed hydration reaction (H₂O/H₂SO₄) of alkenes is not stereospecific because the H and OH atoms can be added to either face of the alkene's double bond. When the reaction occurs, an intermediate carbocation is formed, which is planar. This carbocation can either be attacked by the nucleophile from above or below, resulting in the formation of an equal amount of stereoisomers. Therefore, the correct answer is C. acid-catalyzed hydration reaction (H₂O/H₂SO₄) is not stereospecific.
Learn more about stereospecific at:
https://brainly.com/question/22811170
#SPJ11
Iron has a density of 7.9 g/cm3. What is the mass of a cube of iron with the length of one side equal to 55.0 mm?
Question 3 options:
1.3 × 103 g
2.3 × 10-2 g
4.3 × 102 g
2.1 × 104 g
1.4 g
Answers
The mass of the cube of iron with a side length of 55.0 mm is approximately 1313.6125 grams.
To calculate the mass of a cube of iron, we need to know the density of iron and the length of one side of the cube. Given that the density of iron is 7.9 g/cm^3 and the length of one side of the cube is 55.0 mm, we can proceed with the calculation. First, we need to convert the length of one side from millimeters (mm) to centimeters (cm) since the density is given in grams per cubic centimeter. We divide 55.0 mm by 10 to obtain 5.5 cm.
Next, we can calculate the volume of the cube using the formula V = (side length)^3. Substituting the value of 5.5 cm into the formula, we get V = (5.5 cm)^3 = 166.375 cm^3. Finally, we can calculate the mass of the cube using the formula mass = density × volume. Substituting the values of density (7.9 g/cm^3) and volume (166.375 cm^3), we get mass = 7.9 g/cm^3 × 166.375 cm^3 = 1313.6125 g.
In summary, to calculate the mass of the iron cube, we convert the length from millimeters to centimeters, calculate the volume of the cube, and then multiply it by the density of iron. The resulting mass is approximately 1313.6125 grams.
To learn more about formula mass click here:
brainly.com/question/28647347
#SPJ11
(HELP )Which statement about solids is correct?
Select one:

Answers
Answer:
A. Solids can only change shape if forced to make a change
Explanation:
A solid substance is one of the states of matter.
Matter is anything that has weight and occupies a definite space.
There are three states of matter commonly known: solids, liquids and gases.
Solids have a fixed shape and definite volume. Their crystals are well ordered about a fixed point. It is only when a force is applied that their well ordered arrangement is destroyed and they are forced to make a change.pls help i will mark you the brainlest
Which phrase describes what an object moving at a constant velocity will do? maintain a constant velocity until acted on by another force increase in speed come to a stop, unless it is pushed by another force come to a stop on its own
Answers
Answer:
Maintain constant velocity
Answer: maintain a constant velocity until acted on by another force
Explanation: i took the test
classify each of the following changes as either a physical or a chemical change
1: The addition of the water to quicklime (i.e., the slaking of lime)
2: The melting of candle wax
3: The change in colour of zinc oxide from white to yellow and vice versa during heating and after cooling, respectively
4: The dissolution of common salt
5: The hardening of cement by the absorption of carbon (Iv) oxide
Answers
The changes are classified as follows:
1: Chemical change - The addition of the water to quicklime
2: Physical change - The melting of candle wax
3: Physical change - The change in colour of zinc oxide from white to yellow and vice versa during heating and after cooling, respectively
4: Physical change- The dissolution of common salt
5: Chemical change - The hardening of cement by the absorption of carbon (Iv) oxide
1: The addition of water to quicklime (slaking of lime) is a chemical change. It involves a chemical reaction between calcium oxide (quicklime) and water to form calcium hydroxide (slaked lime). This reaction is exothermic and produces heat.
2: The melting of candle wax is a physical change. It involves a phase transition from a solid state to a liquid state due to the application of heat. The chemical composition of the wax remains unchanged during this process.
3: The change in color of zinc oxide from white to yellow and vice versa during heating and cooling is a physical change. It is a reversible process caused by the alteration of the crystal structure of zinc oxide. The change in color is due to the absorption or release of energy during the heating and cooling processes, respectively.
4: The dissolution of common salt (sodium chloride) is a physical change. It involves the separation of ionic bonds between sodium and chloride ions in the solid salt and their subsequent dispersal in water. The chemical composition of the salt remains the same; it simply forms a homogeneous mixture with water.
5: The hardening of cement by the absorption of carbon dioxide (CO2) is a chemical change. It involves a chemical reaction known as carbonation, where carbon dioxide reacts with the calcium hydroxide in cement to form calcium carbonate. This reaction leads to the formation of new chemical compounds and a change in the structure and properties of the cement, resulting in its hardening or curing process.
For more such questions on quicklime visit:
https://brainly.com/question/15315072
#SPJ8
Atoms of an element, X, have the electronic configuration shown below.
The compound most likely formed with magnesium, Mg, is:
(A) MgX
(B) Mg2X
(C) MgX2
(D) Mg3X2
Answers
The compound most likely formed with magnesium, Mg, is MgX . The correct option is (A) MgX.
The electronic configuration of the element, X is 2,8,7. Since X is located in group 7 of the periodic table, it has 7 electrons in its valence shell. In order to obtain a stable configuration, the atom can either gain one electron to fill the 3rd energy level completely or lose seven electrons to completely empty the 2nd energy level, which is easier. The resulting ion, X-, would have a stable electronic configuration of 2,8. Hence, the compound most likely formed with magnesium, Mg, is MgX .The correct option is (A) MgX.
Explanation: Magnesium is located in group 2 of the periodic table and has two valence electrons in its outermost shell. It loses these two electrons to form Mg2+ ions with stable electronic configurations of 2,8. On the other hand, element X gains an electron to form X- ions with stable electronic configurations of 2,8.The combination of Mg2+ and X- ions results in the formation of the compound MgX with a neutral charge.
Learn more about compound from:
https://brainly.com/question/14782984
#SPJ11
match these items. three-dimensional bonding, hardest natural substance, used as lubricant, nonconductor, weak planar bonds, carbon black or soot
Answers
Answer:
1. three-dimensional bonding
Diamond
2. Hardest natural substance
diamond
3. Used as lubricant
Graphite
4. nonconductor
Diamond
5. Weak, planar bonds
Graphite
6.Carbon black or soot
Amorphous
Explanation;
sorry im late, hopefully this can help somebody :)
A 2.00L flask was filled with 4.00 mol of HI at a certain temperature and given sufficient time to react. At equilibrium the concentration of H2 was 0.400 M. Find the equilibrium concentrations of I2 and HI and then find the Keq at this temperature.
Answers
Answer:
The equilibrium concentration of I₂ is 0.400 M and HI is 1.20 M, the Keq will be 0.112.
Explanation:
Based on the given information, the equilibrium reaction will be,
2HI (g) ⇔ H₂ (g) + I₂ (g)
It is given that 4.00 mol of HI was filled in a flask of 2.00 L, thus, the concentration of HI will be,
= 4.00 mol/2.00 L
= 2.00 mol/L
Based on the reaction, the initial concentration of 2HI is 2.00, H₂ is 0 and I₂ is O. The change in the concentration of 2HI is -x, H₂ is x and I₂ is x. The equilibrium concentration of 2HI will be 0.200-x, H₂ is x and I₂ is x.
It is given that at equilibrium, the concentration of H₂ or x is 0.400 M.
Now the equilibrium concentration of HI will be,
= 2.00 -2x
= 2.00 - 2 × 0.400
= 1.20 M
The equilibrium concentration of I₂ will be,
I₂ = x
= 0.400 M
The equilibrium constant (Keq) will be,
Keq = [H₂] [I₂] / [HI]²
= (0.400) (0.400) / (1.20)²
= 0.112
Thus, the Keq of the reaction will be 0.112.
An indicator is used in a titration toshow when _It does this bychanging color.A there has been a change in temperatureB. to add more waterC. an equal number of moles of acid and base are present

Answers
ANSWER
EXPLANATION
Firstly, we need to define the word titration.
Titration is defined as a technique that is used to determine the known concentration of an unknown solution.
This normally occurs between an acid and a base
During titration, an indicator changes color when equilibrium has been attained between the two solutions. The solutions are normally acid and base. At equilibrium, the number of moles of acid is equal to the number of base.
Therefore, the correct answer is option C
What happens to the following system at equilibrium when it experiences an increase or a decrease in temperature?
H₂O(l) + heat energy ⇌ H₂O(g)
Answers
Similarly, when temperature decreases, you are decreasing the concentration of the ‘heat energy’, meaning the reaction will shift to the left.
which of the following molecules is a hydrocarbon?

Answers
Option C. is a molecule of hydrocarbon.
What are molecules of hydrocarbon?Hydrocarbons are organic compounds made up of only hydrogen and carbon atoms. They are the building blocks of organic chemistry and are the main component of fossil fuels such as coal, oil, and natural gas.
Molecules of hydrocarbons can be simple or complex, and can exist as gases, liquids, or solids. Some common examples of hydrocarbon molecules include methane (CH₄), ethane (C₂H₆), propane (C₃H₈), and butane (C₄H₁₀). Alkanes, alkenes, and alkynes are all types of hydrocarbons that have different chemical and physical properties based on the arrangement of their atoms.
Therefore, the correct answer is as given above. It could then be concluded that only option C is made up of hydrogen and carbon atoms.
learn more about hydrocarbon: https://brainly.com/question/3551546
#SPJ1
In PbO + 2HCl → PbCl2 + H20,13 g of lead oxide is
mixed with 6.4g of hydrochloric acid to produce lead chloride
and water. Which of the following is true?
Answers
Answer:
HCl acts as an excess reagent
Explanation:
The reaction equation is;
PbO + 2HCl → PbCl2 + H20
Number of moles of PbO = 13g/223.2 g/mol = 0.058 moles
Since the mole ratio is 1:1, 0.058 moles of PbCl2 is produced
Number of moles of HCl = 6.4 g/36.5g/mol = 0.175 moles
2 moles of HCl yields 1 mole of PbCl2
0.175 moles yields 0.175 moles * 1/2
= 0.0875 moles of PbCl2
Hence; PbO yields the least number of moles of product so it is the limiting reactant and HCl is the reactant in excess
A farmer has two types of milk, one that is 20% milkfat and one that is 12% milkfat. How much of each is needed to get 20 gallons of 15% milkfat?.
Answers
Two equal expressions combine to make an equation. It takes 7.5 gallons and 12.5 gallons, respectively, of 20% milk and 12% milk to blend them.
How do equations work?When two equal phrases are added together with the aid of the equal sign "=," an equation is created.
Briefing :
Let the amount of 20%milk mixed be represented by x, while the amount of 12% milk mixed is represented by y. Therefore, the two-equation can be written as,
x + y=20 gallons
0.20x + 0.12y = (0.15×20)
Solve the first equation for x, therefore,
x = 20 - y
substitute the value of x in the second equation,
0.20(20-y) + 0.12y = 3
4 - 0.2y +0.12y = 3
-0.08y = -1
y = 12.5 gallons
substitute the value of y in the first equation,
x + 12.5 = 20
x = 7.5 gallons
Hence, the amount of 20% milk and 12% milk that is needed to be mixed is 7.5 gallons and 12.5 gallons, respectively.
To know more about equation visit :
https://brainly.com/question/27805340
#SPJ4
Given that you started with 28.5 g of K3PO4, how many grams of KNO3 can be
produced?
Answers
Mass of KNO₃ : = 40.643 g
Further explanationGiven
28.5 g of K₃PO₄
Required
Mass of KNO₃
Solution
Reaction(Balanced equation) :
2K₃PO₄ + 3 Ca(NO₃)₂ = Ca₃(PO₄)₂ + 6 KNO₃
mol K₃PO₄(MW=212,27 g/mol) :
= mass : MW
= 28.5 : 212,27 g/mol
= 0.134
Mol ratio of K₃PO₄ : KNO₃ = 2 : 6, so mol KNO₃ :
= 6/2 x mol K₃PO₄
= 6/2 x 0.134
= 0.402
Mass of KNO₃ :
= mol x MW KNO₃
= 0.402 x 101,1032 g/mol
= 40.643 g
What relates moles of 1 substance to another in a chemical reaction?
Answers
Answer:
Chemical reactions list reactants and products in molar amounts, not just molecular amounts. We can use the coefficients of a balanced chemical equation to relate moles of one substance in the reaction to moles of other substances
Explanation:
Which organelles release chemicals that break down large food particles into smaller ones?
endoplasmic reticulum
Golgi bodies
vacuoles
lysosomes
Answers
Because they have aromas, compounds with a ring of resonance bonds are called –
A) fragrent compounds
B) pungent molecules
C) aromatic compounds
D) scented structures
Please helppp and no linkss

Answers
Answer:
C I'm pretty sure
Explanation:
uhhhhhhhhh yes
When Newton started studying gravity, the concept of gravity was already in place.
A. True
B. False
Answers
Using the H3O+ or OH- concentrations from your data table above, demonstrate how you would convert each H3O+ (H+ is the same) or OH- solution to pH.

Answers
The procedure by which H₃O⁺ or OH⁻ is converted to pH is to use the given formulas below:
pH = log-[H₃O⁺ ]pOH = log -[OH⁻]pH + pOH = 14What is the relationship between H₃O⁺, OH⁻, and pH?The relationship between H₃O⁺ (hydronium ion), OH⁻ (hydroxide ion), and pH is given below:
pH = log-[H₃O⁺ ]pOH = log -[OH⁻]pH + pOH = 14In an aqueous solution, water molecules ionize resulting in the formation of hydronium ions (H₃O⁺) and hydroxide ions (OH⁻) according to the following equilibrium:
H₂O + H₂O ⇌ H₃O⁺ + OH⁻
Learn more about pH at: https://brainly.com/question/12609985
#SPJ1