Please help
Boiling off a pot of water
A pot containing 500 g of water is brought to a boil.
The latent heat of vaporization is for water HΔv =2260 kJ/kg
How much heat will it take to completely boil the water (turn it all to steam).
Use the equation q = mHΔv
Answers
The equation q = mHΔv is used to calculate the amount of heat required to vaporize a certain amount of substance. In this case, the substance is water and the latent heat of vaporization is 2260 kJ/kg.
The variable q represents the amount of heat required to vaporize the substance, which is measured in joules (J) or kilojoules (kJ). The variable m represents the mass of the substance being vaporized, which is measured in kilograms (kg). Finally, the variable HΔv represents the latent heat of vaporization, which is a property of the substance and is measured in joules per kilogram (J/kg).
When water is heated, it will begin to evaporate, or turn into a gas. This process requires energy in the form of heat. The amount of heat required to vaporize a certain amount of water can be calculated using the equation q = mHΔv. For example, if we want to vaporize 1 kg of water, we can calculate the amount of heat required by multiplying the mass by the latent heat of vaporization:
q = 1 kg x 2260 kJ/kg
q = 2260 kJ
Therefore, it would require 2260 kJ of heat to vaporize 1 kg of water.
In summary, the equation q = mHΔv is a useful tool for calculating the amount of heat required to vaporize a substance, such as water. The latent heat of vaporization is a property of the substance and is required in order to make these calculations.
To know more about vaporization refer here
https://brainly.com/question/14578189#
#SPJ11
Related Questions
which subctant will not conduct electricity a Aluminium b copper c plastic d steel
Answers
Answer:
plastic
Explanation:
plastic doesn't conduct electricity
All of the following contribute to the evolution of a species EXCEPT -
O Respiration
O Adaptations
O Mutations
ONatural selection
Answers
Answer:
Respiration
Explanation:
Respiration is the system of how an animal breathes
Please help I will give brainly.
Thank you so much
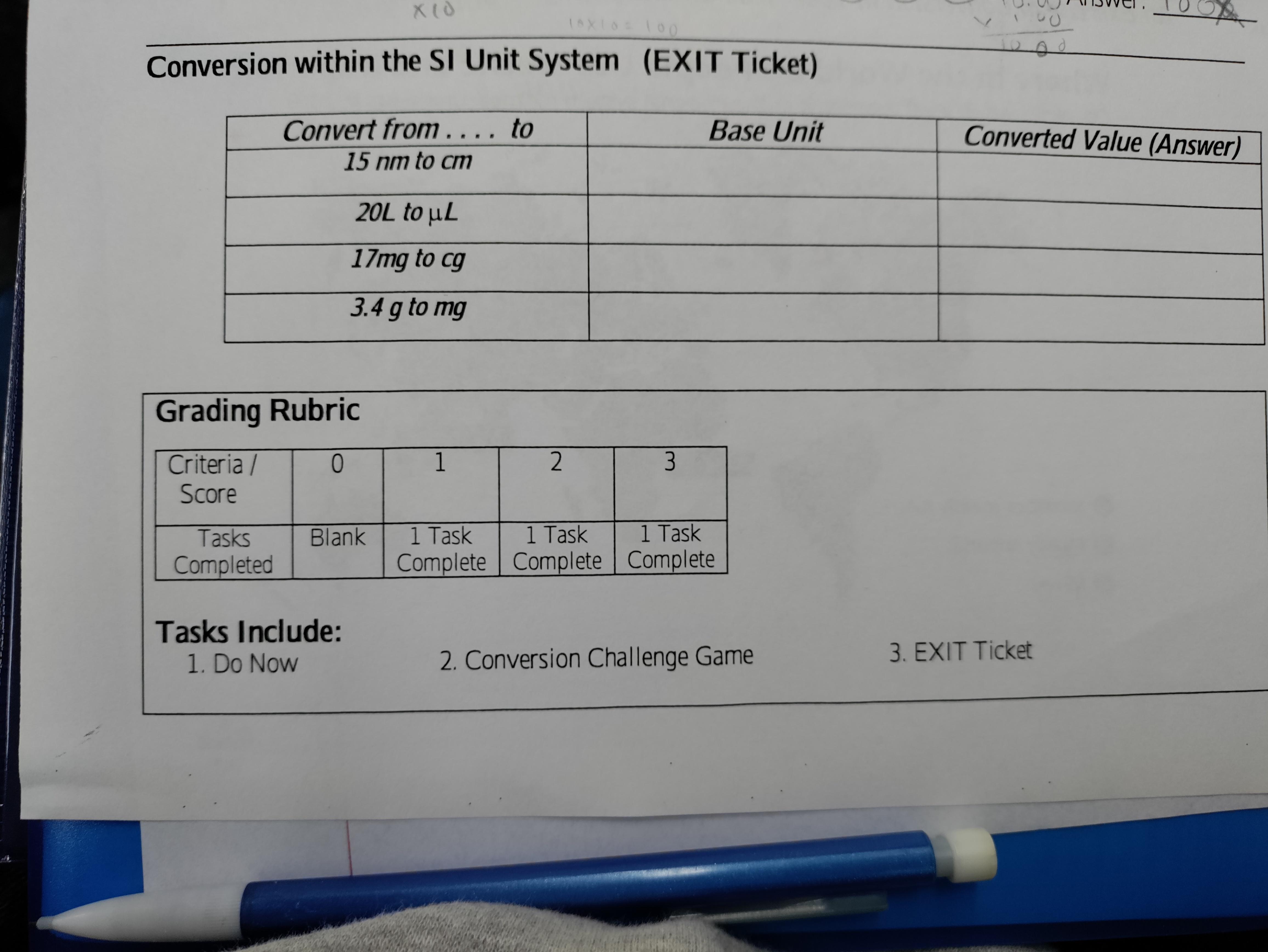
Answers
Answer:
meter. 1.5×10^-6
Litter. 2×10^-5
Kilo Gram. 1.7
Kilo Gram. 3.4 ×10^-3
I hope you understood that and if you don't understand write in comments
what does a proton and neutron have in common
Answers
Answer:Both protons and neutrons have a mass of 1
Explanation:
Answer:
Protons and neutrons are both subatomic particles, which means they are both components of atoms. Protons and neutrons are found in the nucleus of an atom. They both have mass of 1 amu.
Explanation:
Cayden wanted to make some curd. He took some warm milk and added a spoonful of old curd into it. He then kept the milk in his fridge. After 8 hours he took it out. Will he succeed in making curd? Justify your answer. Is there anything you would have done differently or would you follow the same procedure?
Answers
Yes, Cayden will succeed in making curd. We would follow the same procedure to make the curd.
When a spoonful of old curd is added to warm milk, the bacteria present in the curd starts to multiply in the milk. These bacteria convert the lactose (milk sugar) present in the milk into lactic acid, which causes the milk to thicken and form curd. The process of curd formation is called curdling.
When the curdled milk is kept in a fridge, the low temperature inhibits the growth of bacteria, and the curd sets. This is because the lactic acid formed by bacteria during the curdling process makes the milk protein molecules coagulate and form a solid mass.
Therefore, Cayden's procedure of adding a spoonful of old curd to warm milk and keeping it in the fridge is an effective way to make curd.
To know more about bacteria, refer here:
https://brainly.com/question/2501232#
#SPJ11
Escribe verdad o falso: La clorofila capta la energía luminosa del sol. () La fotosíntesis degrada las sustancias orgánicas que nesecitas las células()
Answers
Answer:
La clorofila capta la energía luminosa del sol. (verdadero) La fotosíntesis degrada las sustancias orgánicas que nesecitas las células(falso)
Explanation:
La clorofila es la encargada de dar el pigmento de color verde en las plantas y la que transforma la energia luminica en energia quimica, es por eso que es verdadera.
La fotosintesis NO degrada las sustancias organicas, sino que transforma un sustrato inorganico en una fuente energetica organica para las plantas.
Esto se debe por que las plantas o vegetales son autonomas, es decir que se autoabastecen energeticamente sin necesidad de ingerir alimentos como en el caso de los mamiferos.
SCIENCE, PLEASE HELP.
Which of the following correctly pairs the subatomic particle with its charge? (2 points) Group of answer choices Electron—neutral Electron—positive Neutron—neutral Neutron—positive
Answers
Answer: B
Explanation:
protons are positive,
electrons are negative,
and neutrons are neutral.
the amount of electrons to protons is always the same in a balanced atom.
electrons can be removed creating "ions" which is simply an unbalanced atom. removing protons would result in a different type of atom or element.
Answer:
Neutron - neutral
Explanation:
This was the correct answer for the test I just took.
strength of acids how does the molecular structure of an acid influence its strength
Answers
The strength of acids is influenced by the molecular structure of an acid by polarization of H-A bond and electronic effects like inductive, resonance .
The more the acidic strength, the more is the ability of an acid to donate H+ ions. So, the molecular structure of an acid is very important in determining the strength of the acid. Generally, if the size of an acid molecule increases, the acidic strength increase. If the size of the acid molecule decreases, the acidic strength decreases. There might be few exceptions. In conclusion, the strength of acids is greatly influenced by the molecular structure of an acid.
The following factors affect the acidic strength of acids :
Polarization of the H-A bond: The more polarized the H-A bond, the stronger the acid. This is because a polarized bond means that the electrons are more strongly attracted to one atom (A) than the other (H). This makes it easier for the H atom to be released as a proton (H+). Inductive effect: The inductive effect is a type of electron delocalization that can occur in molecules with multiple atoms. It occurs when electrons are pulled towards atoms that are more electronegative. Inductive effects can weaken the H-A bond, making the acid stronger. Resonance: Resonance is a phenomenon that occurs when a molecule can be represented by multiple Lewis structures that have the same overall electron configuration. Resonance can stabilize a molecule by delocalizing electrons. In the case of acids, resonance can stabilize the conjugate base, making the acid stronger.In general, the key factors that determine the strength of an acid are the presence of polar bonds, the stability of the resulting conjugate base, and the ability to release hydrogen ions (protons).
Thus, the strength of acids is influenced by the molecular structure of an acid by polarization of H-A bond and electronic effects like inductive, resonance .
To learn more about acids :
https://brainly.com/question/15516010
#SPJ11
QUESTION 4 [5 MARKS] Table 5 (a) Assume the consumption function takes the form \( \mathrm{C}=\mathrm{Ca}+(\mathrm{c}) \mathrm{Y} \), then the consumption function based on the information in Table 5
Answers
The consumption function based on the information in Table 5 is as follows: C = 2577 + 0.75Y. It is given, Consumption function, C = Ca + cY Where, Ca is autonomous consumption expenditure, c is marginal propensity to consume (MPC)Y is disposable income
The consumption function based on the information in Table 5 is: Table 5Income(¥ billions)
Consumption(¥ billions)100025020007526000102772750120301.
Write the consumption function in the given format. Ca = Autonomous consumption expenditure c = MPCY = Disposable Income Calculation:
We can obtain the value of Ca as follows: C = Ca + cY
Put the given values, C = 2577Ca + 0.75YAt Y = 1000 billion, C = 2577(1) + 0.75(1000)
= 8327 billion
At Y = 2000 billion, C = 2577(1) + 0.75(2000)
= 13277 billion
At Y = 3000 billion, C = 2577(1) + 0.75(3000)
= 18277 billion
At Y = 4000 billion, C = 2577(1) + 0.75(4000)
= 23277 billion
At Y = 5000 billion, C = 2577(1) + 0.75(5000)
= 28277 billion
Therefore, the consumption function based on the information in Table 5 is as follows: C = 2577 + 0.75Y.
To know more about consumption function, refer
https://brainly.com/question/28145641
#SPJ11
according to this chemical reaction, calculate the number of grams of Fe (55.85 g/mol) produced from 12.57 grams of H2 (2.02 g/mol). Report your answer to the hundredths.
Answers
347.69 grams of Fe are produced from 12.57 grams of\(H_2.\)
The chemical reaction between Fe and H2 is\(:Fe + H_2 -> FeH_2\)
To find out how many grams of Fe are produced from 12.57 grams of H2, we need to use stoichiometry. To do this, we need to first balance the equation. It's already balanced:\(Fe + H_2 -> FeH_2\) .Now, we need to convert 12.57 grams of H2 to moles.
To do this, we need to use the molar mass of \(H_2\), which is 2.02 g/mol:12.57 g.
\(H_2 * (1 mol H_2/2.02 g H_2) = 6.22 mol H_2\)
Now that we know we have 6.22 moles of \(H_2\), we need to figure out how many moles of Fe are needed to react with this amount of \(H_2\).
We can see from the balanced equation that 1 mole of Fe reacts with 1 mole of H2, so we need 6.22 moles of Fe:6.22 mol FeNow that we know how many moles of Fe we need, we can convert that to grams of Fe using the molar mass of Fe, which is 55.85 g/mol:
6.22 mol Fe × (55.85 g Fe/1 mol Fe)
= 347.69 g Fe.
Therefore, 347.69 grams of Fe are produced from 12.57 grams of \(H_2.\)
Know more about here:
#SPJ8
Someone please help will mark as brainliest

Answers
H3AsO4 + 3 I- + 2 H3O+ → H3AsO3 + I3- + H2O
The oxidation of iodide ions by arsenic acid in acidic aqueous solution occurs according to the stoichiometry shown above. The experimental rate law of the reaction is:
Rate = k[H3AsO4] [I-] [H3O+]
Answers
The reaction is in order relative to I in the number 1. when the oxidation of iodide ions by arsenic acid in acidic aqueous solution occurs according to the stoichiometry.
What is rate of reaction?In each of the reactants, the rate law displays the sequence in which a certain reaction occurs. The rate rule is Rate = k [H3AsO4] [I] [H3O+], and it applies to the reaction H3AsO4 + 3 I + 2 H3O+ ------> H3AsO3 + I3 I + H2O.
The reaction is first order with regard to each of the reactants, according to the interpretation of this. Consequently, the reaction is of the order of 1, relative to I.
The pace at which a chemical reaction occurs is known as the reaction rate or rate of reaction, and it is proportional to both the rise in product concentration per unit time and the fall in reactant concentration per unit time. Variable reaction times
For more information on rate of reaction kindly visit to
https://brainly.com/question/13158771
#SPJ4
When the stoichiometric oxidation of iodide ions by arsenic acid in an acidic aqueous solution occurs, the reaction is in order relative to I in number 1.
What is the reaction rate?The rate law illustrates the order in which various reactions take place in each reactant. The reaction H3AsO4 + 3 I + 2 H3O+ follows the rate rule Rate = k [H3AsO4] [I] [H3O+]. H3AsO3 + I3 I + H2O is the equation.
The interpretation of this is that the reaction is first order with respect to each of the reactants. As a result, in relation to I, the reaction is of order 1.
The reaction rate, also known as the rate of reaction, is the rate at which a chemical reaction takes place. It is proportional to both the increase in product concentration per unit of time and the decrease in reactant concentration per unit of time. different reaction times.
To know more about the rate of reaction visit :
https://brainly.com/question/13440548
#SPJ4
Which phrase describes the relative age of a rock?
A uses the law of superposition to determine which rock is older or younger
B can determine the age based on location of a rock within the same layer
C requires only one rock to determine the age
D can provide the exact age of a rock
Answers
Answer:
i think it MIGHT be D
Explanation:
Answer:
b
Explanation:
edge :)
We are studying the ideal gas law. In this discussion, you will be trying your hand at applying one of the ideal gas laws to a real world situation. Consider a situation that involves an ideal gas law and discuss how you would apply your chosen ideal gas law to the situation. Generate an ideal gas law question based on this situation.
Please do not forget to generate a question.
Answers
The ideal gas law, which relates the pressure, volume, temperature, and number of moles of an ideal gas, can be applied to real-world situations. By considering a specific scenario and applying the ideal gas law, we can analyze the behavior of gases and make predictions about their properties.
Let's consider a situation where a scuba diver is exploring underwater at a depth of 30 meters. We can apply the ideal gas law, specifically the form known as Boyle's law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Question: How does the pressure of the gas in the scuba tank change as the diver descends to a depth of 30 meters, assuming the temperature remains constant?
To answer this question, we can use the ideal gas law equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. By keeping the temperature constant, we can observe the relationship between pressure and volume as the diver descends and calculate the change in pressure based on the change in volume.
To learn more about Boyle's law click here:
brainly.com/question/30367133
#SPJ11
How many grams of sodium hydroxide are required to prepare a 200 ml solution of a 10% (weight per volume) solution? (Atomic weights: Na = 23; 0 = 16; H = 1)
Answers
Given that we want to prepare a 10% solution of sodium hydroxide in 200 mL, we need to calculate the mass of sodium hydroxide required to make this solution.
We can use the following formula to calculate the mass of solute required to make a given volume and concentration of solution:
mass of solute = volume of solution x concentration of solution x density of soluteFirst, let's calculate the density of sodium hydroxide.The density of solid NaOH is 2.13 g/mL. So, the density of sodium hydroxide solution is:
Density = 2.13 g/mLNow, let's substitute the given values into the formula to calculate the mass of sodium hydroxide required:mass of solute = 200 mL x 0.10 x 2.13 g/mL= 4.26 gTherefore, 4.26 grams of sodium hydroxide are required to prepare a 200 mL solution of a 10% (weight per volume) solution.About Sodium hydroxideSodium hydroxide, also known as lye and caustic soda or caustic soda, is an inorganic compound with the chemical formula NaOH. This compound is an ionic compound in the form of a white solid composed of the sodium cation Na⁺ and the hydroxide anion OH⁻. Sodium hydroxide is a building block that can also be found in detergents and oil stain removers. We use it to make products clean better by influencing the formula molecules, so they work better together.
Learn More About Sodium hydroxide at https://brainly.com/question/25866669
#SPJ11
Which Base In An Anticodon Will Pair With The Base Adenine (A) In A Codon? A. Cylosine (C) B. Guanine (G) C, Uracil (U) D. Thymine (T)
Answers
The base in an anticodon that will pair with the base adenine (A) in a codon is uracil (U).
When there is DNA replication, the genetic code from the DNA molecule is transferred to the messenger RNA molecule. The messenger RNA molecule contains codons, which are three bases long sequences. These sequences are complementary to the three-base long anticodons found in transfer RNA.The base pairing is done according to the complementary base pairing rule: cytosine (C) pairs with guanine (G), and adenine (A) pairs with thymine (T) in DNA or uracil (U) in RNA. In the process of protein synthesis, the anticodon found in transfer RNA pairs with the codon found in messenger RNA. Since adenine pairs with uracil in RNA, the base in an anticodon that will pair with the base adenine (A) in a codon is uracil (U).Therefore, option C, Uracil (U), is the correct answer.
To know more about uracil click on below link:
https://brainly.com/question/2368011#
#SPJ11
100 cm³ of a gas at 27°C is cooled to 20°C at constant pressure .Calculate the volume of gas at 20°C.
Answers
According to Charle's law, the volume of the given mass of a gas is directly proportional to its absolute temperature provided that the pressure is constant. Mathemically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V}{T} \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where;
V1 and V2 are the initial and final volume of the gas
T1 and T2 are the initial and final temperatures of the gas (in Kelvin)
Given the following parameters:
\(\begin{gathered} V_1=100\operatorname{cm}^3 \\ T_1=27^0C=27+273=300K \\ T_2=20^0C=20+273=293K \\ V_2=\text{?} \end{gathered}\)Substitute the given parameters into the formula;
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1}^{} \\ V_2=\frac{100\times293}{300} \\ V_2=\frac{29300}{300} \\ V_2=\frac{293}{3} \\ V_2=97.67\operatorname{cm}^3 \end{gathered}\)Therefore the volume of the gas at 20°C is approximately 97.67cm³
A modest level of ethanol enters the human circulation naturally from __________, equivalent to a fraction of a drink per day.
Answers
intestinal flora
A modest level of ethanol enters the human circulation naturally from intestinal flora, equivalent to a fraction of a drink per day.
What is intestinal flora?inside the intestines are bacteria and other living things. They aid in food digestion. Intestinal flora produces vitamins like biotin and vitamin K. Also known as intestinal microflora, microflora, and gut flora.Are intestinal flora good?Humans have 100 trillion viable bacteria in their intestines. The intestinal flora is the collective name for these living bacteria, which account for 30% of the faeces bulk. The intestinal flora contains both useful and dangerous microbes. They are evenly distributed and the good bacteria take center stage in healthy persons.What purpose does the gut flora serve?Intestinal microflora primarily serves two purposes:
(1) metabolic processes that result in the absorption of nutrients and energy; and (2) host defense against invasion by alien microbes.The immune system's development and balance depend heavily on intestinal microorganisms.
To learn more about intestinal flora visit:
https://brainly.com/question/12993029
#SPJ4
Which of the following is an oxidation-reduction reaction? KOH + HNO3 → H2O + KNO3 CaCl2 + Na2SO4 → CaSO4 + 2NaCl AgNO3 + NaCl → AgCl + NaNO3 Al2(SO4) 3 + 6KOH → 2Al(OH)3 + 3K2SO4 N2 + O2 → 2NO
Answers
Answer:
N2 + O2 → 2NO is an oxidation-reduction reaction.
Explanation:
An oxidation-reduction reaction (also know as Redox reaction) is a type of chemical reaction that involves a transfer of electrons between two species. It occurs when the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.
We will consider the equations one after the other.
KOH + HNO3 → H2O + KNO3Oxidation numbers of the individual element on the reactant side:
KOH : K = +1, O = -2, H = +1
HNO3 : H = +1, N = +5, O = -2
Oxidation numbers of the individual element on the product side:
H2O: H = +1, O = -2
KNO3: K = +1, N = +5, O = -2
There is no change in the oxidation numbers, hence, it is not an oxidation-reduction reaction.
CaCl2 + Na2SO4 → CaSO4 + 2NaClOxidation numbers of the individual element on the reactant side:
CaCl2 : Ca = +2, Cl = -1
Na2SO4: Na = +1, S = +6, O = -2
Oxidation numbers of the individual element on the product side:
CaSO4: Ca = +2, S = +6, O = -2
NaCl: Na = +1, Cl = -1
There is no change in the oxidation numbers, hence, it is not an oxidation-reduction reaction.
AgNO3 + NaCl → AgCl + NaNO3Oxidation numbers of the individual element on the reactant side:
AgNO3: Ag = +1, N = +5, O = -2
NaCl: Na = +1, Cl = -1
Oxidation numbers of the individual element on the product side:
AgCl: Ag = +1, Cl = -1
NaNO3: Na: +1, N = +5, O = -2
There is no change in the oxidation numbers, hence, it is not an oxidation-reduction reaction.
Al2(SO4) 3 + 6KOH → 2Al(OH)3 + 3K2SO4Oxidation numbers of the individual element on the reactant side:
Al2(SO4)3: Al =+3, S = +6, O = -2
KOH: K = +1, O = -2, H = +1
Oxidation numbers of the individual element on the product side:
Al(OH)3: Al =+3, O = -2, H = +1
K2SO4: K = +1, S = +6, O = -2
There is no change in the oxidation numbers, hence, it is not an oxidation-reduction reaction.
N2 + O2 → 2NOOxidation numbers of the individual element on the reactant side:
N2: N = 0
O2: O = 0
Oxidation numbers of the individual element on the product side:
NO: N = +2, O = -2
There are changes in the oxidation numbers, hence, it is an oxidation-reduction reaction.
The following is an oxidation-reduction reaction - N2 + O2 → 2NO
Oxidation numbers represent the potential charge of an atom in its ionic state. If the oxidation number decreases in a reaction, it is reduced. If an atom's oxidation number increases, it is oxidized.
CaCl2 + Na2SO4 → CaSO4 + 2NaCl is a double displacement reaction KOH + HNO3 → H2O + KNO3 is acid-base neutralization reaction AgNO3 + NaCl → AgCl + NaNO3 is double displacement reaction Al2(SO4) 3 + 6KOH → 2Al(OH)3 + 3K2SO4 is double displacement reactionAll three reaction mention above is not oxidation-reduction reaction as each atom has the same oxidation number in these reactions. N2 + O2 → 2NO is oxidation-reduction reaction.= > Oxidation state of N in the reactant is 0
=> Oxidation state of N in the product is +2
So, N is oxidized
=> Oxidation state of O in the reactant is 0
=> Oxidation state of O in the product is -2
So, O is reduced
Thus, the following is an oxidation-reduction reaction - N2 + O2 → 2NO
Learn more:
https://brainly.com/question/11672211
What drugs are calcium channel blockers?
Answers
Answer:
Examples of calcium channel blockers include:
Amlodipine (Norvasc)
Diltiazem (Cardizem, Tiazac, others)
Felodipine.
Isradipine.
Nicardipine.
Nifedipine (Procardia)
Nisoldipine (Sular)
Verapamil (Calan SR, Verelan)
How much 0.50mbacl2 solution, in liters, will completely precipitate the ba2 in 1.0lof0.15molal2(so4)3 solution?
Answers
The volume of 0.50M BaCl2 solution needed to react with the Ba2+ ions is 0.44 L (440 mL) of 0.50M BaCl2 solution.
To determine the amount of 0.50M BaCl2 solution needed to completely precipitate Ba2+ in 1.0L of 0.15 molal Al2(SO4)3 solution, we need to consider the stoichiometry of the reaction.
From the balanced equation:
BaCl2(aq) + Al2(SO4)3(aq) -> BaSO4(s) + 2AlCl3(aq)
We can see that one mole of BaCl2 reacts with one mole of BaSO4. Since BaCl2 is the limiting reactant, we need to calculate the number of moles of Ba2+ in the Al2(SO4)3 solution.
0.15 molal solution means 0.15 moles of Al2(SO4)3 dissolved in 1 kg of solvent (water). The molar mass of Al2(SO4)3 is 342 g/mol, so we have:
0.15 moles x 342 g/mol = 51.3 g Al2(SO4)3 in 1 kg of solvent
Now, we need to convert this mass of Al2(SO4)3 to moles of Ba2+ ions. Since the molar mass of BaSO4 is 233.4 g/mol and it has a 1:1 stoichiometry with Ba2+, we have:
51.3 g / 233.4 g/mol = 0.22 moles of Ba2+ ions
Finally, we can calculate the volume of 0.50M BaCl2 solution needed to react with the Ba2+ ions:
0.22 moles / 0.50 mol/L = 0.44 L (440 mL) of 0.50M BaCl2 solution.
To know more about solution visit:-
https://brainly.com/question/1616939
#SPJ11
How many moles of sucrose are in 5.25x10^29 sucrose molecules?

Answers
Answer:
8.72×10^5
Explanation:
Answered it in the comments! ^^
2. Combine lead (II) nitrate and potassium iodide solutions.
Pb(NO3)2+ Kl →
2. Combine lead (II) nitrate and potassium iodide solutions.
Pb(NO3)2+ Kl →
3. Combine magnesium metal and hydrochloric acid solution.
Mg + HCl →
4. Electrolysis (splitting) of water.
H2O →
5. Burning magnesium.
Mg + O2 →
Answers
Answer:
Pb(NO3)2+ 2Kl →PbI2 + 2KNO3
Mg + 2HCl → MgCl2 + H2
4H2O → 4H^+ + 4OH^-
2Mg + O2 → 2MgO
Explanation:
This question has to do with the balancing of chemical reaction equations. The general rule for balancing chemical reaction equation is that the number of atoms of each element on the left hand side of the reaction equation must be equal to the number of atoms of the same element on the right hand side of the reaction equation.
This principle was followed in balancing each reaction equation above. For instance, in the burning of magnesium, there are two atoms of both magnesium and oxygen on either side of the reaction equation.
Choose an example of a reaction to which Markovnikov's rule applies.
O CH₂=CH-CH2-CH3 + HBr CH₂ Br=CH2-CH2-CH3
O CH,=CH-CH, CH3 + HBr → CHg =CHBr–CH2–CH3
O CH,=CH-CH,—CH, + HBr → CH,Br–CHBr–CH2–CH, + HBr CH₂Br-CH2-CH2-CH3
O CH₂=CH-CH2-CH3 O CH,=CH-CH2–CH3 + HBr → CH3–CHBr–CH2–CH3
Answers
The example of a reaction to which Markovnikov's rule applies is: CH₂=CH-CH₂-CH₃ + HBr → CH₂Br-CH₂-CH₂-CH₃
In this reaction, the hydrogen atom from HBr adds to the carbon atom with the fewer alkyl substituents (less substituted carbon), while the bromine atom adds to the carbon atom with more alkyl substituents (more substituted carbon). This follows Markovnikov's rule, which states that in the addition of a protic acid (such as HBr) to an asymmetrically substituted alkene, the hydrogen atom adds to the less substituted carbon and the other atom adds to the more substituted carbon.
To learn more about Markovnikov's, https://brainly.com/question/32087294
#SPJ11
Please Help me it’s balancing equations

Answers
Answer:
Blank 1: 1
Blank 2: 1
Blank 3: 2
Explanation:
I think the question is like this __Na2O +___H2O =___NaOH
I used "á" this word as equal sign. Is there any wrong with that?
If lava from volcanoes moves slowly enough that people can get away from it, why are volcanoes dangerous?
Answers
Answer:
I think this is the right answer, I researched this. I am not sure if this is the right answer.
Lava flows rarely kill people because they move slowly enough for people to get out of their way. If magma is thick and sticky, gases cannot escape easily. Pressure builds up until the gases escape violently and explode. ... Explosive volcanic eruptions can be dangerous and deadly.
Explanation:
A filament bulb is labelled ‘2.5 V, 1.25 W'. How much energy will be transferred to it when it is connected to a 2.5 V battery for one minute?
Answers
To calculate the amount of energy transferred to the filament bulb, we can use the power, voltage, and time involved in the circuit. The given information states that the bulb is labeled as '2.5 V, 1.25 W'.
To start, we can use the formula P = IV, where P represents the power, I represents the current, and V represents the voltage. Since the resistance of the bulb is not provided, we cannot directly use the formula P = V² / R. However, we can derive the value of current using I = V / R, where R is the resistance.
By substituting I = V / R into the equation P = IV, we obtain P = V² / R. Now, we have the power value, which is 1.25 W. The bulb is connected to a 2.5 V battery, so we can use P = IV to find the current, I. Substituting P = 1.25 W and V = 2.5 V into the equation, we get I = P / V = 1.25 / 2.5 = 0.5 A.
Now that we know the current flowing through the bulb is 0.5 A, we can calculate the energy transferred using the formula E = Pt, where E represents energy, P represents power, and t represents time. Given that the bulb is connected for 1 minute (60 seconds), we can calculate E = Pt = 1.25 W × 60 s = 75 J (Joules).
Therefore, the amount of energy transferred to the filament bulb when it is connected to a 2.5 V battery for one minute is 75 Joules.
To Learn more about filament bulb. Click this!
brainly.com/question/22940744
#SPJ11
The pOH of a solution of 0.15 M HCl (aq) would be what?

Answers
Answer:
13.18
Explanation:
We'll begin by calculating the hydrogen ion concentration, [H⁺] in the solution. This can be obtained as follow:
HCl (aq) —> H⁺(aq) + Cl¯(aq)
From the balanced equation above,
1 mole of HCl dissociate to produce 1 mole of H⁺.
Therefore, 0.15 M HCl will also dissociate to produce 0.15 M H⁺.
Thus, the concentration of the hydrogen ion, [H⁺] in the solution is 0.15 M.
Next, we shall determine the pH of the solution. This can be obtained as follow:
Concentration of the hydrogen ion, [H⁺] = 0.15 M.
pH =?
pH = – Log[H⁺]
pH = – Log 0.15
pH = 0.82
Finally, we shall determine the pOH of the solution. This can be obtained as follow:
pH = 0.82
pOH =?
pH + pOH = 14
0.82 + pOH = 14
Collect like terms
pOH = 14 – 0.82
pOH = 13.18
Therefore, the pOH of the solution is 13.18
The pOH of the solution is 13.18 when a solution of 0.15 M HCl (aq) is present.
What is pH?The potential of hydrogen; a measure of the acidity or alkalinity of a solution equal to the common logarithm of the reciprocal of the concentration of hydrogen ions in moles per cubic decimetre of solution.
Concentration of the hydrogen ion, [H⁺] = 0.15 M.
pH =?
pH = – Log[H⁺]
pH = – Log 0.15
pH = 0.82
Finally, we shall determine the pOH of the solution. This can be obtained as follow:
pH = 0.82
pOH =?
pH + pOH = 14
0.82 + pOH = 14
pOH = 14 – 0.82
pOH = 13.18
Therefore, the pOH of the solution is 13.18
Learn more about pH here:
https://brainly.com/question/15231899
#SPJ5
I need help with my science project someone help me answer it!

Answers
Answer:
food
Explanation:
If you have 9 moles of cacl2 and all the na3po4 i needed, how many moles of nacl could you produce?
Answers
If you have 9 moles of CaCl₂ and all the Na₃PO₄i needed 18 moles of NaCl
In the chemical reaction here
CaCl₂ + Na₃PO₄ → Ca₃(PO₄)₂ + NaCl if we balance this chemical reaction then
3CaCl₂ + 2Na₃PO₄ → Ca₃(PO₄)₂ + 6NaCl then Ca=1×3=3
,Cl=2×3=6,
Na=3,PO₄=1,
all this are in reactant Ca=3, Cl=1, Na=1, PO₄=2 all this are in product so NaCl produce 18 moles.
Know more about CaCl₂ and Na₃PO₄ moles .
https://brainly.com/question/28499515
#SPJ4