predict the major product for each of the following reactions
Answers
4-Chlorobut-1-ene with HBr (a)
In this reaction, an alkene is present. Thus, there will be an addition reaction in which the most substituted carbon will get the addition of the nucleophile "". Here, carbon 3 results in 3-bromo-1-chlorobutane.
1-Chlorobut-1-ene with HBr (b)
We additionally have an alkene in this reaction. Thus, there will be an addition reaction in which the most substituted carbon will get the addition of the nucleophile "". Here, carbon 2 creates 2-bromo-1-chlorobutane.
(c) H2O, H+, and 4,4-Dimethylcyclopentene
In this reaction, an alkene is present. Thus, there will be an addition reaction in which the most substituted carbon will get the addition of the nucleophile "". 3,3-dimethylcyclopentan-1-ol is produced in this instance from carbon 3.
Propyne with 2HCl (d)
In this reaction, an alkyne is present. We will therefore experience an additional reaction.
To learn more about reaction please click on below link
https://brainly.com/question/28984750
#SPJ4
Related Questions
Sand particles have density equal to 0.70 g/mL. A teacher ordered a 22 kg bag of sand from the scientific supply company. Sand plus air (as ordered) takes up 1.3 times as much volume as the sand particles alone. What is the volume in cubic feet (ft3) of sand plus air that the teacher ordered?
I need so much help with Dimensional Analysis aaaa
Answers
The volume of sand plus air that the teacher order is 14.4 x 10^4 ft3.
What is volume?
Volume is defined as the space occupied within the boundaries of an object in three dimensional space. It is also known as capacity of the object.
The SI unit of volume is liter.
To calculate the volume of sand :
Density = mass / volume
As density is given 0.70g/ml and mass of sand is 22kg then,
Volume = mass/ density
= 22 / 0.7g/ml = 31.43 g/ml or cm3
As sand plus air is 1.3 time much as volume of sand alone,
Then 31.43g/ml = 31.43 x 1.3 = 40.86 g/ml or cm3
40.86 cm3 is equal to 14.4 x 10^4 ft3
Thus, the volume of sand plus air that the teacher order is 14.4 x 10^4 ft3.
To learn more about volume, refer to the link below:
https://brainly.com/question/13338592
#SPJ1
How many moles are in 15g of k2O? The molar mass of k2o is 94. 2g/mol
Answers
According to the question of mass, 0.159 moles moles are in 15g of k₂O.
What is mass?Mass is a scientific concept that describes the amount of matter contained in a given object or material. It is measured in kilograms (kg) and is different from weight, which is measured in Newtons. Mass is the measure of an object’s inertia and its resistance to acceleration when a force is applied. In other words, it is the amount of matter in an object, regardless of its shape or size. Mass can be determined using a variety of instruments or machines, like a balance or a scale. Mass is a fundamental property of matter and is used to calculate the weight of an object. Mass is a key concept in physics, chemistry, and engineering.
15g of k₂O is equal to 0.159 moles. This can be calculated by dividing 15g by the molar mass of k₂O (94.2g/mol): 15g/94.2g/mol = 0.159 moles.
To learn more about mass
https://brainly.com/question/837939
#SPJ4
You have three elements, A, B, and C, with the following electronegativity values:
Scoi
A = 0.9
B = 3.0
C = 3.5
You react the elements to form the substances AB, AC, and BC. Answer the following questions:
What type of substance is AB? What types of bonds are present? Explain your answer.
What type of substance is AC? What types of bonds are present? Explain your answer.
What type of substance is BC? What types of bonds are present? Explain your answer.
If any of the substances are ionic compounds, which element is the cation and which is the anion?
Explain your answer.
Answers
AB is an ionic Compound , BC is covalent Compound , AC is an Ionic Compound.
What is Electronegativity ?It is the tendency of an atom in which molecule is usually attract towards the shared pair of electrons which generally is known as electronegativity. As we move across a period i.e from left to right. Here the nuclear charge increases and the atomic size decreases, hence the value of electronegativity increases across a period according to the modern periodic table.Atomic number increases as we move down the group.The nuclear charge also increases but it's effect increase in nuclear charge is overcome with the help of addition of one shell. the value of electronegativity decreases while moving down the group.Now,
A = 0.9
B = 3.0
C = 3.5
AB is an ionic compound. If the difference of electronegativity between two atoms is more than 1.7 , then they will form ionic compound. Here A is Cation and B is Anion .
Electronegativity difference between A and C = 3.5 - 0.9 = 2.6Hence AC is an ionic compound.
A is Cation and B is Anion .
Electronegativity difference between B and C = 3.5 - 3.30 = 0.5Hence BC is a covalent Compound.
Thus , from the above conclusion we can say that, AB is an ionic Compound , BC is covalent Compound , AC is an Ionic Compound.
Learn more about Ionic Compound here: https://brainly.com/question/26126708
#SPJ9
What is irony bring out the elements of irony in the story?.
Answers
Irony: a situation in which there is a contrast between expectation and reality.Irony can take many different forms.
The verbal irony, dramatic irony, and situational irony are the three types .Irony occurs in literature AND in life whenever a person says something or does something that departs from what they (or we) expect them to say or do.
Irony in the story 'Dusk':
Ironically, the main character, who thinks he is a good character judge, is duped by the young man who sat next to him on the park seat.
According to Gortsby, darkness is a time when dejected and shady people emerge.
The term irony has its roots in the Greek comic character Eiron, a clever underdog who by his wit repeatedly triumphs over the boastful character Alazon. The Socratic irony of the Platonic dialogues derives from this comic origin.
FIND MORE:-https://brainly.in/question/28085927
#SPJ4
How many moles of hydrogen are required to react with 4.6 x 10 22 molecules of nitrogen?
Answers
Answer: 6 moles
Take a look at the balanced chemical equation for this synthesis reaction
N 2(g] + 3 H 2(g] → 2 NH 3(g]
Notice that you have a 1:3 mole ratio between nitrogen gas and hydrogen gas. This means that, regardless of how many moles of nitrogen gas you have, the reaction will always consume twice as many moles of hydrogen gas.
So, if you have 2 moles of nitrogen taking part in the reaction, you will need
2 moles N 2 ⋅ 3 moles H 2 /1 mole N 2 = 6 moles H 2
When is a rock considered an ore?
Check all that apply.
-when it occurs in sufficient amounts to be used to build roads and buildings
-only when it contains lead
-when it contains at least one metallic mineral in sufficient amounts to be extracted profitably
-only when it contains iron
-when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably
Answers
The correct statements are that a rock is considered an ore when it contains at least one metallic mineral in sufficient amounts to be extracted profitably and when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
Step-by-step explanation:
When it occurs in sufficient amounts to be used to build roads and buildings: This statement is incorrect. Rocks that are used for construction purposes, such as limestone or sandstone, are not considered ores unless they also contain economically valuable minerals.
Only when it contains lead: This statement is incorrect. Ores are not limited to containing only lead. Ores can contain various metallic minerals, not just lead.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably: This statement is correct. Ores are rocks that contain valuable metallic minerals in concentrations that make extraction economically feasible. These minerals can include gold, silver, copper, aluminum, zinc, and many others.
Only when it contains iron: This statement is incorrect. While iron ores are commonly known and widely used, ores are not limited to containing only iron. There are numerous other metallic minerals that can be extracted profitably from rocks.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably: This statement is correct. Fluorite and sulfur are examples of non-metallic minerals that can be extracted profitably from rocks, and if a rock contains these minerals in sufficient quantities, it can be considered an ore.
learn more about from metallic mineral the given link:
https://brainly.com/question/89259
#SPJ11
How many molecules are in 12.8 moles of CO2?
A. 563 molecules
B. 0.291 molecules
C. 2.13 x 10^{-23} molecules
D. 7.71 x 10^{24} molecules
Answers
Answer: D. 7.71 x 10^24
Explanation:
12.8 moles CO2 (6.02 * 10^23 molecules / 1 mol) = 7.7 * 10^24 molecules :)
Which would probably be the most effective way to reduce the amount of methane released into the atmosphere?
answer choices
a. Reducing the amount of gasoline we use
b. Making sure to prevent forest fires
c. Reducing the number of cows we raise for meat
d. Reducing the amount of electricity we use
Answers
Reducing the number of cows we raise for meat would probably be the most effective way to reduce the amount of methane released into the atmosphere
How to reduce methaneMethane is a potent greenhouse gas that contributes to global warming, so reducing its emissions is important in mitigating climate change. Here are some ways to reduce methane:
Reduce or eliminate the use of fossil fuels: Methane is released during the extraction, production, and transport of fossil fuels such as coal, oil, and natural gas. By using renewable energy sources like solar or wind power instead, we can reduce our dependence on fossil fuels and the associated methane emissions.
Reduce waste: Methane is produced when organic waste decomposes in landfills, wastewater treatment plants, and agricultural operations. By reducing the amount of waste we produce and by implementing better waste management practices, such as composting and anaerobic digestion, we can reduce methane emissions.
Use methane as a fuel: Methane can be captured and used as a fuel for electricity generation, heating, and transportation. This is known as biogas, and it can be produced from sources such as livestock manure, agricultural waste, and wastewater. Using biogas as a fuel reduces the amount of methane released into the atmosphere while providing a renewable energy source.
Read more on methane here:https://brainly.com/question/25649765
#SPJ1
According to the law of conversion of mass in a chemical equation
Answers
In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction. If we account for all reactants and products in a chemical reaction, the total mass will be the same at any point in time in any closed system.
Why is human waste hazardous when it enters the water system? for London and Ancient Rome?
I will mark as brainliest
Answers
........,,,,,,?!!mm........
Answers
Answer:
añadir agua a la mezcla para disolver la sal. Después la arena se separa del agua salada por filtración. Finalmente, el agua salada se deja evaporar.
Explanation:
:u
An+impure+sample+of+the+same+hydrocarbon+is+found+to+have+a+%+by+mass+of+carbon+of+80.00+%.+is+this+observation+consistent+with+an+impurity+that+contains+no+carbon?+explain+your+answer
Answers
No, the observation of an 80.00% mass percentage of carbon in an impure sample of the same hydrocarbon is not consistent with an impurity that contains no carbon.
Since the impure sample of the hydrocarbon is found to have a mass percentage of carbon of 80.00%, it indicates that carbon is a major component of the sample. The high percentage suggests that the impurity is not solely responsible for the carbon content in the sample. If the impurity contained no carbon, the mass percentage of carbon in the sample would be significantly lower.
The observed high carbon content suggests that the impurity, if present, is likely to contribute to the carbon content of the sample. It could be a different compound or a carbon-containing impurity mixed with the hydrocarbon. The presence of carbon in the impure sample could arise from various sources such as incomplete purification, contamination during handling, or the inherent composition of the original hydrocarbon source.
To determine the exact nature of the impurity and its contribution to the carbon content, further analysis and characterization techniques would be required. These may include spectroscopic methods, elemental analysis, or chromatographic techniques to identify and quantify the impurity components.
In summary, the high mass percentage of carbon in the impure sample suggests that the impurity itself is likely to contain carbon, indicating that the observation is not consistent with an impurity that contains no carbon.
Learn more about carbon
brainly.com/question/13046593
#SPJ11
Solve for missing values using the ideal gas law formula:
1. 10°C, 5. 5 L, 2 mol, __ atm. What is the atm?
2. __ °C, 8. 3 L, 5 mol, 1. 8 atm. What is the temperature in celsius?
3. 12°C, 3. 4 L, __ mol, 1. 2 atm. What is the mole?
Answers
The ideal gas law formula is used to determine the missing values in questions. When dealing with problems that require solving for missing values using the ideal gas law formula, always ensure that all values are expressed in the correct units and temperature is converted to kelvin.
The ideal gas law formula is represented as PV = nRT, where P represents pressure, V represents volume, n represents the number of moles of gas, T represents the temperature in kelvin, and R represents the universal gas constant. Solve for missing values using the ideal gas law formula:1. 10°C, 5. 5 L, 2 mol, __ atm.The temperature must be converted to kelvin first: T(K) = T(°C) + 273.15K = 10°C + 273.15 = 283.15KPV = nRT
Rearrange the equation to isolate P: P = nRT / V
Substitute the given values:
P = (2 mol)(0.0821 L•atm/mol•K)(283.15K) / 5.5 L
: P = 8.28 atm
2. __ °C, 8. 3 L, 5 mol, 1. 8 atm.The equation PV = nRT can be rearranged to T = PV / nRThe temperature must be converted to kelvin first: T(K) = T(°C) + 273.15T = PV / nR
Substitute the given values: T = (1.8 atm)(8.3 L) / (5 mol)(0.0821 L•atm/mol•K)T(K) = T +
: T = 332 K or 59°C
The temperature must be converted to kelvin first:
T(K) = T(°C) + 273.15K
= 12°C + 273.15
= 285.15
KPV = nRT
Solve for n by rearranging the equation: n = PV / RT
Substitute the given values: n = (1.2 atm)(3.4 L) / (0.0821 L•atm/mol•K)(285.15K): n = 0.141 mol
The ideal gas law formula is used to determine the missing values in questions. When dealing with problems that require solving for missing values using the ideal gas law formula, always ensure that all values are expressed in the correct units and temperature is converted to kelvin.
To know more about ideal gas law visit:
brainly.com/question/30458409
#SPJ11
A worm is shown in the picture.
What kind of worm is it?
a planarian
a leech
a fluke
a tapeworm

Answers
Answer:
It's a Planarian
Explanation:
Answer above
Answer:
A
Explanation:
if the exponent applied to the concentration of a in a rate law is equal to one, then that reaction must be:
Answers
If the exponent applied to the concentration of A in a rate law is equal to one, then that reaction must be a first-order reaction. In a rate law, the exponent represents the order of the reaction with respect to a specific reactant.
In a first-order reaction, the rate of the reaction is directly proportional to the concentration of A. This means that if the concentration of A doubles, the rate of the reaction will also double. Mathematically, the rate law for a first-order reaction can be represented as follows:
rate = k[A]
Here, [A] represents the concentration of A, k is the rate constant, and the exponent 1 indicates that the reaction is first-order with respect to A.
To further illustrate, let's consider an example. Suppose we have a reaction where the rate law is given as rate = k[A]. If the concentration of A is 2 M, and the rate of the reaction is 0.1 M/s, we can determine the rate constant as follows:
0.1 M/s = k * 2 M
Simplifying the equation, we find k = 0.05 1/s.
In conclusion, if the exponent applied to the concentration of A in a rate law is equal to one, it signifies a first-order reaction, where the rate is directly proportional to the concentration of A.
To know more about rate law visit:-
https://brainly.com/question/30379408
#SPJ11
Una especie que se aparea en la superficie y luego deposita sus huevos en el agua para ser rodeadas por los espermas, el de
Answers
The species would be an anuran (an amphibian)
Which type of species is it?We need to answer this in English.
Here we want to find "A species that mates on the surface and then deposits its eggs in the water to be surrounded by sperm"
This would be known as a species of amphibian called an anuran, specifically a frog or toad.
Frogs and toads belong to the order Anura and have a characteristic reproductive cycle that involves external fertilization. During reproduction, the male releases his sperm into the water, and the female deposits her eggs in the water as well. The eggs are externally fertilized by sperm that swim around them. This process is known as external or external fertilization.
Learn more about eggs:
https://brainly.com/question/1151355
#SPJ1
why do the aquatic plants not break down by water current?
Answers
15 POINTS
Terms such as boil, freeze, condense, vaporize, or melt in chemistry generally refer to a ______ in matter.
Answers
Answer:
change
Explanation:
These terms refer to the change into the different states of matter.
BRAINLIEST.
Tell me honnnnnn.
An object has a mass of 100kg and a volume of 10m^3.
What is its density?
SHOW WORK FOR FULL CREDIT
Answers
Answer:
Mass = 100g
Volume = 10 cm³
Density = Mass/Volume
= 100/10
= 10 cm-³
The mixture which has same composition throughout is called(a) homogeneous
(b) heterogeneous
(c)none
Answers
The mixture that has the same composition throughout is called a (a) homogeneous mixture. In a homogeneous mixture, the components are uniformly distributed at a molecular or microscopic level, resulting in a uniform appearance and properties throughout the mixture.
This means that no matter where you sample the mixture, you will find the same proportions of its components.
An example of a homogeneous mixture is a solution, such as sugar dissolved in water. The sugar molecules are uniformly dispersed in the water, creating a homogeneous mixture where the composition is the same regardless of where you sample the solution.
In contrast, a heterogeneous mixture is one in which the components are not uniformly distributed and can be visually distinguished. Examples of heterogeneous mixtures include a mixture of oil and water, or a salad dressing with visible layers of oil and vinegar.
Therefore, the correct answer is (a) homogeneous.
To know more about the homogeneous mixture refer here,
https://brainly.com/question/30587533#
#SPJ11
A car with a mass of 1,000 kg is moving at a speed of 20 m/s. What is the car's kinetic energy?
Answers
The answer would be 200000 J. the equation for kinetic energy is 1/2 mass times velocity squared. 1/2 of 1,000 is 500. and 20*20 is 400. So, multiply 400 by 500, and that gives you your answer,
Forming ionic compounds:
Write the formula unit for the ionic compound
1. Na and O
2. K and S
3. Br and Ca
4. Ba and N
5. Al and P
6. I and Al
7. F and Sr
8. Li and Cl
Answers
Answer:
Name Formula and Charge Name Formula and Charge
ammonium NH4+ hydroxide OH−
acetate C2H3O2−, or CH3COO− nitrate NO3−
bicarbonate (hydrogen carbonate) HCO3− nitrite NO2−
bisulfate (hydrogen sulfate) HSO4− peroxide O22−
carbonate CO32− perchlorate ClO4−
chlorate ClO3− phosphate PO43−
chromate CrO42− sulfate SO42−
cyanide CN− sulfite SO32−
dichromate Cr2O72− triiodide I3−
Explanation:
permanent dipole moment between a partially positive and a partially negative end of 2 molecules
Answers
As a result, a permanent dipole is added to the O-H bond. The oxygen atom is somewhat negatively charged, whereas the hydrogen atom is partially positively charged.
What method is used to determine a molecule's permanent dipole moment?
The ratio of the charge's magnitude to the space between the positive and negative charge centers is known as the dipole moment. The Greek letter " stands for it. It is expressed as a number of Debye units, or "D." 1 D equals 3.33564 10-30 C.m, where C stands for Coulomb and m for a metre. Forces between dipoles. Dipole-dipole forces act as an attracting force between the positive and negative ends of two polar molecules. Dipole-dipole forces have molecular energies of between 5 and 20 kJ. The ratio of the charge's magnitude to the space between the positive and negative charge centers is known as the dipole moment.
The Greek letter " stands for it. It is expressed as a number of Debye units, or "D." 1 D equals 3.33564 10-30 C.m, where C stands for Coulomb and m for a metre.
To learn more about dipole moment refers to:
brainly.com/question/11626115
#SPJ4
what is the osmotic pressure (at 25°c) of seawater? it contains approximately 35.0 grams of nacl per liter. (seawater contains other stuff, but we'll ignore it.)
Answers
Therefore, the osmotic pressure of seawater, which contains approximately 35.0 grams of NaCl per liter, at 25°C is approximately 1498.59 Pa.
how to calculate the osmotic pressure ?
The osmotic pressure of seawater can be calculated using the Van't Hoff equation, which relates the osmotic pressure of a solution to its concentration and the temperature. The equation is given by:
Π = MRT
where M is the molarity of the solute, R is the ideal gas constant, and T is the temperature in kelvins. The molar mass of NaCl is 58.44 g/mol, so 35 g of NaCl in a liter of water corresponds to a molarity of:
M = 35 g / 58.44 g/mol = 0.599 mol/L
Converting the temperature from degrees Celsius to kelvins gives:
T = 25°C + 273.15 = 298.15 K
Substituting the values into the Van't Hoff equation gives:
Π = 0.599 mol/L * 8.31 J/(mol*K) * 298.15 K
Π = 1498.59 Pa
Therefore, the osmotic pressure of seawater, which contains approximately 35.0 grams of NaCl per liter, at 25°C is approximately 1498.59 Pa.
To learn more about osmotic pressure follow the given link: https://brainly.com/question/29714361
#SPJ1
WORTH 100 POINTS !!!
What does it mean when a reaction reaches equilibrium?
Answers
Explanation:
A reaction at equilibrium essentially means that the number of reactants and products is constant (but not necessarily equal) and continue to be constant until a change is imposed on the system.
Which statement is true of rectangles Y and Z? Rectangle Y has a length of 6 and width of 4. Rectangle Z has a length of 4 and width of 3. They are congruent because their corresponding angles are congruent. They are similar because their corresponding angles are congruent. They are similar because their corresponding side lengths are proportional. They are not similar because their corresponding side lengths are not proportional.
Answers
Answer:
b
Explanation:
Answer:
ita b
Explanation:
trust fam
The __________ is the main control center for the autonomic nervous system. A. Forebrain B. Thalamus C. Hypothalamus D. Cerebrum Please select the best answer from the choices provided A B C D.
Answers
Answer:
its Hypothalamus
Explanation:
the mass of the sun
what is the mass of the sun
Answers
Answer:
1.989 × 10^30 kg
Explanation:
Hope this helps! :3
☁️ Answer ☁️
A solar mass is the mass of the sun. Or, more precisely, it's 1.989 x 10^30 kilograms — about 333,000 Earths. Astronomers use a solar mass as a basic unit of mass.
Dear fool, you will always be a fool.
Happy April Fools Day.
Hope it helps.
Have a nice day noona/hyung
NEED HELP ASAP!!! TYSM!!!! What are the coefficients when the following equations are balanced?
__CH4 + __O2 --> __CO2 + __H2O
__Fe + __O2 --> __Fe2O3
Answers
4Fe+3O2–>2Fe2O3
Level 1
What is the first step in completing the
scientific method?
A: Experimentation
B: Forming a hypothesis
C: Analyzing data
D: Making observations
Q1
Level 1
Q2
Which of the following is not true about
a hypothesis?
A: It is an explanation for an observation
B: It must restate the question
1. C. It is testable
D. It can be written as an if/then statement,
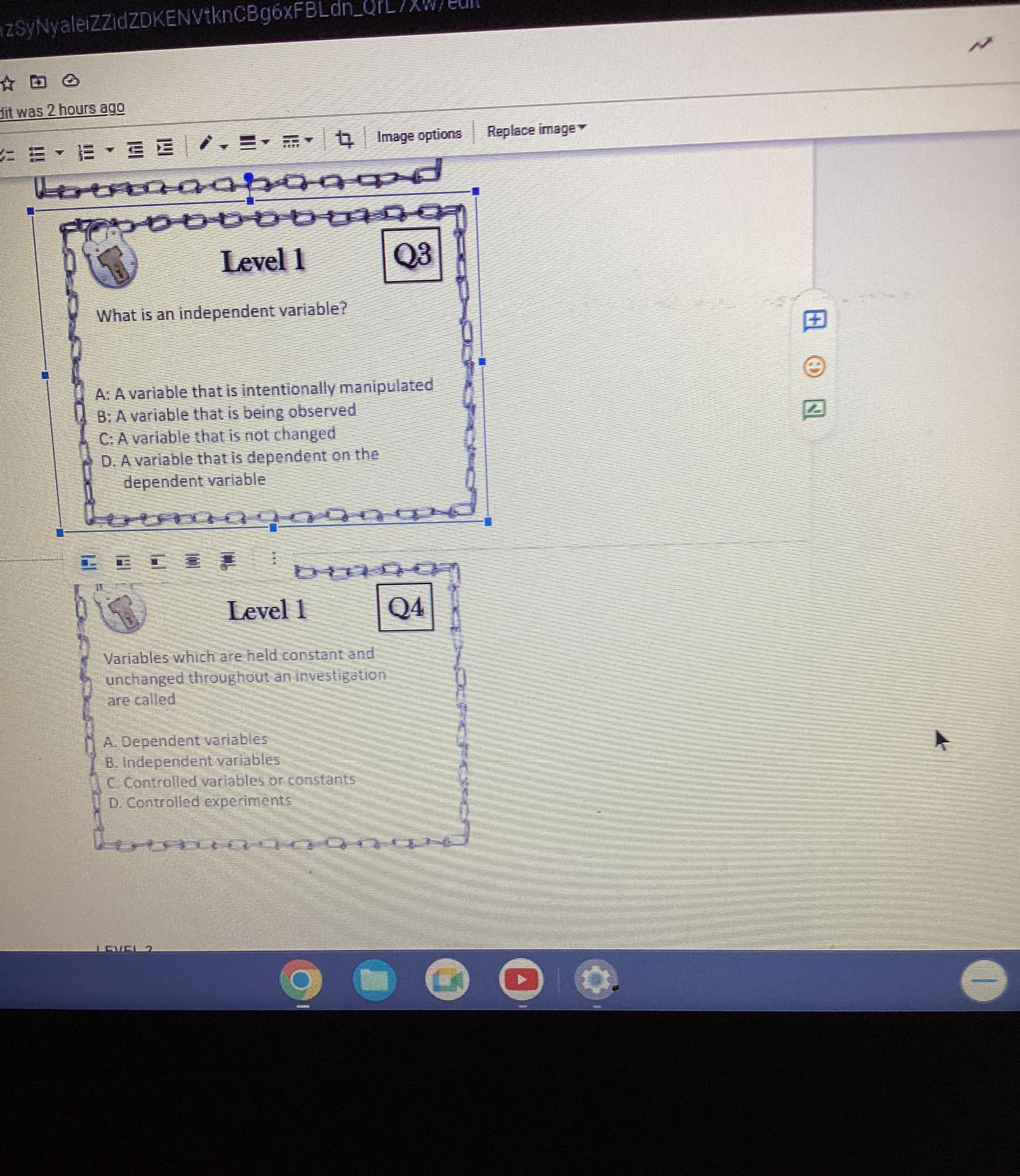
Answers
Answer: Level 1's answer is D/ Making observations. The second one is it must restate the question/ B. No. 3:variable (often denoted by x ) whose variation does not depend on that of another. No.4 IS Controlled Vari
Explanation: