Q 9 The table lists the steps to clean water for drinking purpose. 1. Adding chlorine tablets to the water.2. Pouring the water through the candle filter 3. freezing the water 4. Reusing the water in multiple household activities Which set of steps would help to provide water fit for drinking?
(a) 1 and 2
(b) 2 and 3
(c) 3 and 4
(d) 4 and 1
Answers
Answer:
1 and 2
Explanation:
when we r adding chlorine to water
the water is clean
Related Questions
A 25.0 mL sample of 0.125 molL−1 pyridine (Kb=1.7×10−9) is titrated with 0.100 molL−1HCl Calculate the pH at 40 mL of added acid.
Answers
The pH of added acid at 40 mL is 1.87.
Given,
Concentration of pyridine = 0.125 M
Concentration of HCl = 0.1 M
Volume of pyridine = 25 mL
The millimoles of HCl is calculated as follows:
mmol of HCl = Molarity × Volume of HCl
= 0.1 M × 40 mL
= 4 mmol
The millimoles of pyridine is calculated as follows:
mmol of pyridine = Molarity × Volume of HCl
= 0.125 M × 25 mL
= 3.125 mmol
The concentration of excess HCl is calculated as follows:
Concentration of excess HCl = Millimoles / Total volume
= (4.0 - 3.125) mmol / (40 + 25) mL
= 0.0135 M
The pH of added acid is calculated as shown below:
pH = - log [H⁺]
= - log (0.0135)
= 1.87
Hence, the pH of added acid is 1.87.
Learn more about pH from the link given below.
https://brainly.com/question/16232412
#SPJ1
A 29.3-g sample of an alloy at 93.00 °C is placed into 50.0 g of water at 22.00 °C in an insulated coffee-cup calorimeter with a heat capacity of 9.20
K. If the final temperature of the system is 31.10 °C, what is the specific heat capacity of the alloy?
Answers
The specific heat capacity of the alloy is 0.120 J/g°C.
What is Specific heat capacity?
Specific heat capacity, also known as specific heat, is the amount of heat required to raise the temperature of a unit mass (usually one gram) of a substance by one degree Celsius or Kelvin. Each substance has its own specific heat capacity, which depends on its chemical composition and physical state. The SI unit of specific heat is joules per gram per degree Celsius (J/g°C).
First, we need to calculate the heat absorbed by the water:
q_water = m_water * C_water * ∆T
where m_water is the mass of water, C_water is the specific heat capacity of water, and ∆T is the change in temperature of the water.
m_water = 50.0 g
C_water = 4.18 J/g°C
∆T = 31.10°C - 22.00°C = 9.10°C
q_water = (50.0 g) * (4.18 J/g°C) * (9.10°C) = 1911.5 J
Next, we need to calculate the heat released by the alloy:
q_alloy = - q_water
Since the calorimeter is insulated, the heat lost by the alloy is equal to the heat gained by the water.
q_alloy = m_alloy * C_alloy * ∆T
where m_alloy is the mass of the alloy, C_alloy is the specific heat capacity of the alloy, and ∆T is the change in temperature of the alloy.
m_alloy = 29.3 g
∆T = 31.10°C - 93.00°C = -61.90°C (note the negative sign)
q_alloy = (29.3 g) * C_alloy * (-61.90°C)
Finally, we can solve for the specific heat capacity of the alloy:
C_alloy = - q_water / (m_alloy * ∆T)
C_alloy = - (1911.5 J) / ((29.3 g) * (-61.90°C))
C_alloy = 0.120 J/g°C
Therefore, the specific heat capacity of the alloy is 0.120 J/g°C.
Learn more about Specific heat capacity from given link
https://brainly.com/question/27991746
#SPJ1
Calculate the N/Z ratio for elements with atomic numbers 104 through 109. Are they in the belt of stability? Are they stable? How do you know?
Answers
The ratio of neutrons to protons, or the N/Z ratio, plays a crucial role in determining a nucleus' stability. The range of N/Z ratios in which nuclei are stable is generally referred to as the belt of stability.
How can you tell whether a substance is stable or unstable?If the forces between the constituents of the nucleus are equal, an atom is stable. If these forces are out of balance or if the nucleus has an excessive amount of internal energy, an atom is unstable (radioactive).
Z = 104 for Rutherfordium, element 104. The isotopes 261Rf and 262Rf, having masses of 261 and 262, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
261Rf: N/Z = (261-104)/157 = 1.08
262Rf: N/Z = (262-104)/158 = 1.09
These N/Z ratios are a little bit higher than the average belt of stability values, which are about 1.0 for heavy nuclei. These isotopes are thought to be reasonably stable because they are close enough.
Z = 109 for Meitnerium, element 109. The isotopes 278Mt and 282Mt, with masses of 278 and 282, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
278Mt: N/Z
To know more about neutrons visit:-
https://brainly.com/question/29248303
#SPJ1
PLEASE HELPPP
UNIT TEST Chemical Reactions - Part 1 Which equation is balanced? CP+20 - PO₂ 2P+ 0; --P.₂O. 4P + 50., --2P.0. 4P - 20. - 27.0. - →
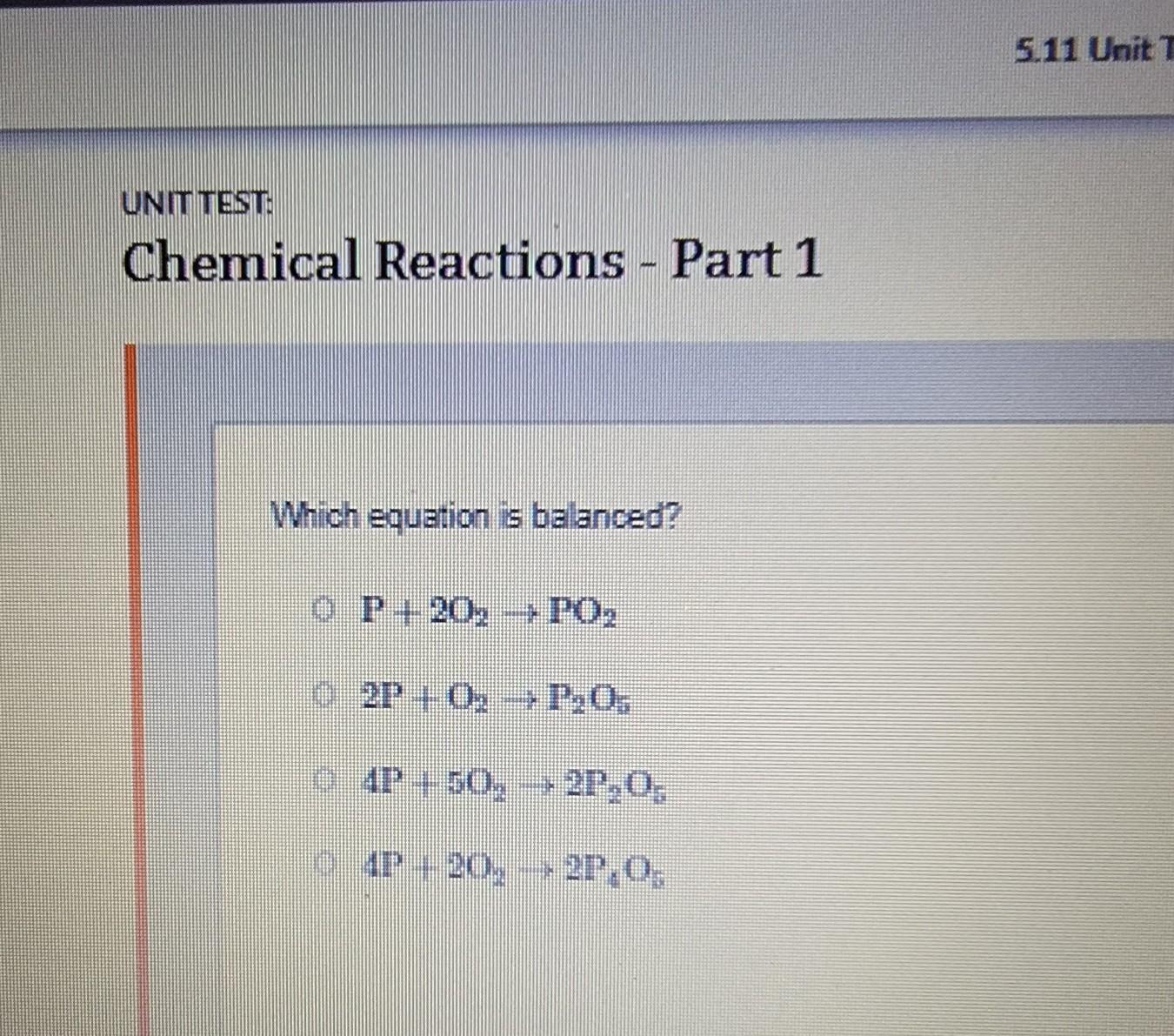
Answers
Answer:
2P+ 02 → P205Explanation:
I Thinks it B!!!
but please don't take my word i'm not really good at math.When our calculator math provides the value 0.0021471, but we need to record the value with only three significant figures, what would we record?
Answers
The number that is given as digits is established using significant figures. A meaningful representation of numbers is carried by these digits. Frequently, significant digits are employed in place of figures.
Thus, By counting all of the values beginning with the first non-zero digit on the left, we may determine the number of significant digits.
The crucial or important digits that accurately represent the meaning of a certain number are known as the significant figures of that number.
6.658, for instance, has four significant digits. These huge amounts give the numbers accuracy. Additionally, they are known as significant digits.
Thus, The number that is given as digits is established using significant figures. A meaningful representation of numbers is carried by these digits. Frequently, significant digits are employed in place of figures.
Learn more about Significant figures, refer to the link:
https://brainly.com/question/23396760
#SPJ1
How many moles are present
in 3.25 x 1024 atoms P?
A. 0.185 mole
C. 5.40 moles
B. 1.85 moles
D. 1.96 x 1048 moles
Answers
Answer:
1 mole =6.02×10^23atoms
3.25×10^24atoms
3.25×10^24/6.02×10^23
5.40 moles
C is correct option
Calculate the mass of calcium carbonate produced if 15.2 mL of 0.306 M K2CO3 is reacted with 17.0 mL of 0.295 M CaCl2.
Answers
Answer:
\(m_{CaCO_3}=0.465g\)
Explanation:
Hello!
In this case, since the reaction between two aqueous solutions may turn out in the production of a solid precipitate, for potassium carbonate and calcium chloride, calcium carbonate is precipitated out as shown below:
\(CaCl_2(aq)+K_2CO_3(aq)\rightarrow 2KCl(aq)+CaCO_3(s)\)
Now, since the two reactants are in a 1:1 mole ratio, we infer they react in the same proportion, thus we compute the reacting moles, considering the used volumes of those molar solutions:
\(n_{K_2CO_3}=0.0152L*0.306mol/L=0.00465mol\\\\n_{CaCl_2}=0.0170*0.295mol/L=0.00502mol\)
Thus, since just 0.00465 mol out of 0.00502 moles of calcium chloride are consumed, the potassium carbonate is the limiting reactant, therefore the mass of yielded calcium carbonate (molar mass = 100.09 g/mol) is:
\(m_{CaCO_3}=0.00465molK_2CO_3*\frac{1molCaCO_3}{1molK_2CO_3} *\frac{100.09gCaCO_3}{1molCaCO_3}\\\\m_{CaCO_3}=0.465g\)
Best regards!
Describe the function of a cilia.
Answers
Answer:
hope it helps..
Explanation:
'Motile' (or moving) cilia are found in the lungs, respiratory tract and middle ear. ... These cilia have a rhythmic waving or beating motion. They work, for instance, to keep the airways clear of mucus and dirt, allowing us to breathe easily and without irritation.
Answer: to keep airway clear of mucus and dirt allowing us to breathe easy
Explanation:
arrange the following in order of ionization energy, Na, Na+, C, O
Answers
Answer:
Na+, O, C, Na
(Na+ has more ionization energy and Na has less ionization energy)
As the temperature increases from 0°C to 25°C the amount of NH3 that can be dissolved in 100 grams of water.
A) decreases by 10 grams
B) decreases by 40 grams
C) increases by 10 grams
D) increases by 40 grams
Answers
Answer:
decreases by 10 gram
Explanation:
At 23°C, 85.0 grams of NaNO3(s) are dissolved in 100. grams of H2O(l).
Based on Table G, determine the additional mass of NaNO3(s) that must be dissolved to
saturate the solution at 23°C.
Answers
5g
Anything between 4 and 6 is ok.
The additional mass of solute to be added will be determined by the mass of solute required to saturate a solution of NaNO3 in 100. grams of H2O.
A saturated solution is one that contains just as much solute as it can normally hold at a given temperature. We should now that when additional solute is added to a saturated solution, the additional solute does not dissolve.
The table is not presented hence the question is incomplete. However, the additional mass of solute to be added will be determined by the mass of solute required to saturate a solution of NaNO3 in 100. grams of H2O.
Learn more about solubility: https://brainly.com/question/953809
When a strong acid or base is added to water it...
Answers
When a strong acid or base is added to water, the pH will change dramatically.
Strong AcidA strong acid is one that is completely dissociated or ionized in an aqueous solution. This means it gives off the greatest number of hydrogen ions or protons when placed in a solution. Examples of strong acid are HCl, HBr, H2SO4, HNO4. These acids when placed in water, produces greatest amount of hydrogen ions. The pH value changes drastically. Any that has very high concentration of hydrogen and ion is acidic.
Also when base is added to water, the pH of water will increase above 7 and become basic. The pH of water is 7, but when base is added to it increases above 7.
Base is any solution that is slippery to touch in water solution, changes color, react with acid to form salt and change red litmus paper to blue.
Learn about acid and base in water solution here
https://brainly.com/question/27915098
#SPJ1
Which example has particles that can be drawn closer to occupy smaller volume? a. fruit juice b. block of wood c. air inside the syringe d. ice cube
Answers
The capacity of an object is measured by its volume. For instance, a cup's capacity is stated to be 100 ml if it can hold 100 ml of water in its brim. Here ice cube has particles that can be drawn closer to occupy smaller volume. The correct option is D.
Due to the strong intermolecular interactions, the solid molecules are very near to one another. Solids have a low volume and a high density as a result. Additionally, the solid molecules cannot be easily crushed due to the narrow intermolecular distance.
So here ice cube has small volume.
Thus the correct option is D.
To know more about volume, visit;
https://brainly.com/question/13807002
#SPJ1
Think of other muscles in your body, besides your heart, that work without you thinking about them. How do the functions of these muscies differ from ones you consciously control?
Answers
Answer:
Smooth Muscles - Smooth muscles are special muscles that don't connect to bones, but control organs within our body. These muscles work without us having to think about them.Explanation:
Sana makatulong heart and follow po and pa brainliest thnks(PLEASE HELP)"The most abundant isotope of Hydrogen has a mass of 1 amu. Therefore, the average atomic mass on the periodic table for Hydrogen will be 1 amu." Using the terms isotopes, abundance, average atomic mass and weighted mass to explain why this misconception is incorrect and why the abundance(amount) of each isotope of the element affect the average atomic mass.
Answers
The statement the most abundant isotope of Hydrogen has a mass of 1 amu is wrong.
What is the average atomic mass?The average atomic mass could be obtained as the sum of the products of the percentage abundance of each of the hydrogen isotopes and the mass of the isotope.
We know that every element has isotopes. In every sample of the atom, there are various isotopes of the element. Thus the average atomic mass must take into account the masses of all these isotopes.
As such, the statement the most abundant isotope of Hydrogen has a mass of 1 amu is wrong.
Learn ore about atomic mass:https://brainly.com/question/17067547
#SPJ1
What is the main molecule that provides the energy to produce ATP?
Answers
Answer: glucose
Explanation:
There are ____ unpaired up electrons in [Ni(NH3)6]3+
Answers
There are 7 unpaired electrons in [Ni(NH\(_3\))\(_6\)]\(_3\)⁺. The elementary electric charge of the electron is a negative one, making it a subatomic particle.
The elementary electric charge of the electron is a negative one, making it a subatomic particle. Due to their lack of components or substructure, electrons, which are part for the lepton particle family's first generation, are typically regarded to be elementary particles.
The mass of an electron is roughly 1/1836 that of a proton. The electron has a half-integer inherent angular momentum (spin) that is described in terms of the shortened Planck constant,, among its quantum mechanical features. There are 7 unpaired electrons in [Ni(NH\(_3\))\(_6\)]\(_3\)⁺.
To know more about electrons, here:
https://brainly.com/question/1255220
#SPJ1
Calculate the pH and the pOH of an aqueous solution that is 0.020 M in HCl(aq) and 0.085 M in HBr(aq) at 25 °C.
Answers
pH = -log(0.115) <<< use a calculator
pOH = 14 - pH <<< just subtract
pH(HCl) = 1.5 => pOH = 12.5
pH(HBr) = 1.1 => pOH = 12.9
< Question 27 of 27 > You decide it is time to clean your pool since summer is quickly approach chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppin. you send a sample of pool water to a chemist for analysis of the Cl₂ conte 3.71 × 10–5 M. Convert the concentration of Cl, to parts per million (ppm). Macmillan Learning concentration:
Answers
To kill bacteria, chlorine is added to the water. However, it does not function immediately away and kill CDC.
Thus, When handled correctly, free chlorine* can destroy the majority of bacteria in a matter of minutes.
The CDC advises maintaining a pH of 7.2–7.8 and free chlorine levels of at least 1 ppm in swimming pools and 3 ppm in hot tubs and spas.
The CDC advises a pH of 7.2–7.8 and a free accessible chlorine content of at least 2 ppm in swimming pools when using cyanuric acid, a chlorine stabilizer, or chlorine products containing cyanuric acid (for instance, products generally known as dichlor or trichlor. The CDC advises against using cyanuric acid or chlorine products containing it in hot tubs or spas.
Thus, To kill bacteria, chlorine is added to the water. However, it does not function immediately away and kill CDC.
Learn more about Chlorine, refer to the link:
https://brainly.com/question/19460448
#SPJ1
Drag the tiles to the correct boxes to complete the pairs.
Match the descriptions with the types of blas.
selection bias
expectation bias
confirmation bias
contextual bias
paying more attention to evidence that
confirms one's hypothesis and ignoring
evidence that may discount it
to be swayed from one's conclusion by
additional information
coming to a conclusion before all the
evidence has been processed and therefore
unconsciously disregarding evidence to the
contrary
Answers
Answer:
Explanation:
here's the answer hope it helps! :)

please help quick will give brainliest
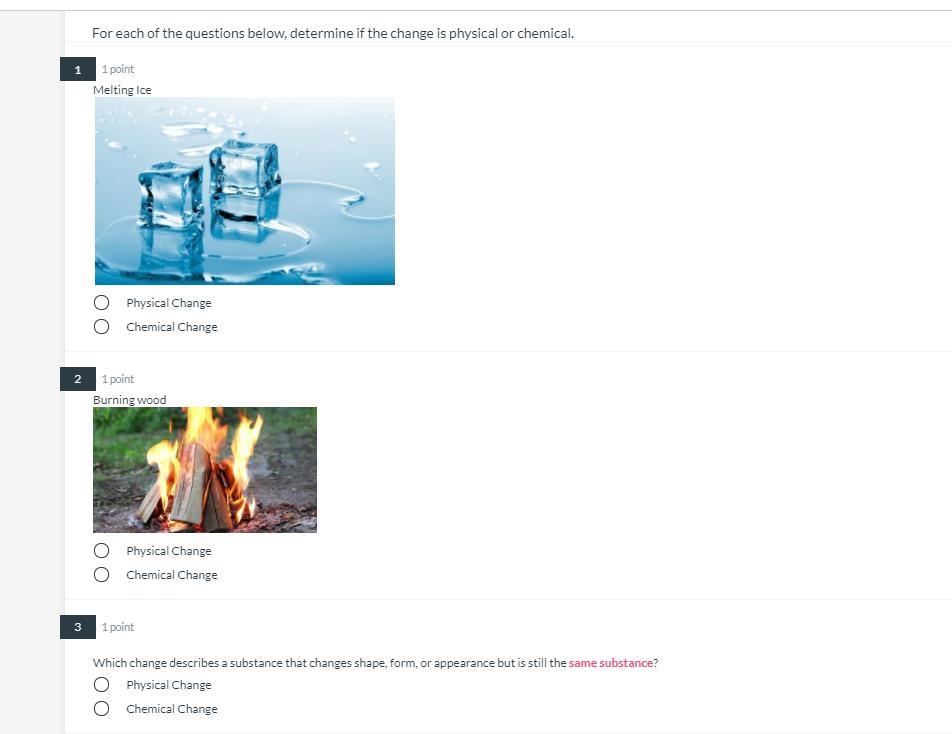
Answers
Answer:
I think it's Physical, Chemical, then Physical
Explanation:
I hope I'm right, those answers sound about right to me
*
Malleability refers to the ability of a metal to...
be drawn into a wire
conduct electricity
be melted down to create alloys
O be hammered into shapes

Answers
Answer:
It would be to be hammered into shapes because malleability is how flexible a metal is.
[24 points] A sample of soil has a total volume of 205 cm3. The soil mass when saturated is 361 g. A specific yield test was conducted on the soil by allowing the sample to drain for 24 hours. After drainage the sample mass was 295 g. The soil was then dried and weighed 284 g. What are the specific yield [8 points], specific retention [8 points], and porosity [8 points] of the sample
Answers
Answer:
Follows are the solution to the given question:
Explanation:
Dry Soil weight = solid soil weight = \(284 \ grams\)
solid soil volume =\(205 \ cc\)
saturated mass soil = \(361 \ g\)
The weight of the soil after drainage is =\(295 \ g\)
Water weight for soil saturation = \((361-284) = 77 \ g\)
Water volume required for soil saturation =\(\frac{77}{1} = 77 \ cc\)
Sample volume of water: \(= \frac{\text{water density}}{\text{water density input}}\)
\(= 361- 295 \\\\ = 66 \ cc\)
Soil water retained volume = (draining field weight - dry soil weight)
\(= 295 - 284 \\\\ = 11 \ cc.\)
\(\text{POROSITY}= \frac{\text{Vehicle volume}}{\text{total volume Soil}}\)
\(= \frac{77}{(205 + 77)} \\\\= \frac{77}{(282)} \\\\ = 27.30 \%\)
(Its saturated water volume is equal to the volume of voids)
\(\text{YIELD SPECIFIC} = \frac{\text{Soil water volume}}{\text{Soil volume total}}\)
\(= \frac{66}{(205+77)}\\\\= \frac{66}{(282)}\\\\=0.2340\\\\ = 0.23\)
\(\text{Specific Retention}= \frac{\text{Volume of soil water}}{\text{Total soil volume}}\)
\(= \frac{11}{282} \\\\= 0.0390 \\\\ = 0.04\)
How much will the temperature a 60 g sample of iron (specific heat = 0.214 cal/g/*C) change if 2000 calories are added to it?
Answers
Answer:
37.36 degrees Celsius
Explanation:
The temperature change of a substance can be calculated using the formula:
ΔT = Q / (m * Cp)
Where:
ΔT is the change in temperature (in degrees Celsius)
Q is the heat added or removed (in calories)
m is the mass of the substance (in grams)
Cp is the specific heat capacity of the substance (in calories per gram per degree Celsius)
For a 60g sample of iron with a specific heat of 0.214 cal/g*C and 2000 calories added to it, the temperature change would be:
ΔT = 2000 / (60 * 0.214) = 37.36 degrees Celsius
33) Which is the correct name for the molecule depicted below?
A. 2-isopropyl-2,3,4-trimethylbutane
B. 2-isopropyl-2,3-dimethylpentane
C. 2,3,3,4-tetramethylhexane
D. 1,1,2,2,3-pentamethylpentane
E. None of these choices is correct.

Answers
Answer:
C. 2,3,3,4-tetramethylhexane
Explanation:
family of macromolecules are composed of carbon, hydrogen and oxygen in a 1:2:1 ratio and includes sugars, starches, glycogen, and cellulose. multiple choice question. protein lipid nucleic acid carbohydrate need help? review these concept resources.
Answers
Macromolecules that are composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio and include sugars, starches, glycogen, and cellulose is carbohydrate.
Macromolecules: Carbohydrate, Lipid, Protein, and Nucleic acidMacromolecules are molecules that are made of small molecules (monomers).
There are 4 types of macromolecules: carbohydrate, lipid, protein, and nucleic acid.
Carbohydrate is made up of monomer glucose. Each compound is composed of carbon, hydrogen, and oxygen in a 1: 2: 1 ratio. Carbohydrate is classified into polysaccharide (glycogen, starch, cellulose), disaccharide (lactose, sucrose, maltose), and monosaccharides (glucose, galactose, and fructose). Protein is composed of monomer amino acids. The elements of protein are carbon, hydrogen, oxygen, and nitrogen, also has -COOH, -NH2, and R groups.Lipids contain fatty acid and glycerol chains. The element that makes up the lipid are carbon, hydrogen, and oxygen (not in a 1: 2: 1 ratio). For example fats, and oils.Nucleic acid contains elements carbon, hydrogen, oxygen, nitrogen, and phosphorus. For example DNA, RNAThus, sugars, starches, glycogen, and cellulose are included in carbohydrates. The ratio of elements in carbohydrates is 1:2:1.
Learn more about macromolecules by clicking this link :
https://brainly.com/question/17637031
#SPJ4
5 gallons to ? L
1gal = 3.785 L
Answers
A gallon is a common volume measurement unit for measuring liquids and occasionally dry things. 5 gallon is equal to 18.925 L.
Thus, The US liquid gallon, US dry gallon, and Imperial gallon are the three different sorts of gallons. To measure and store commodities like fuel, oil, milk, paint, and many other things, these three types are frequently employed.
The English Parliament created the gallon in 1696 to be used for measuring dry goods. After gaining independence, the US adopted the Winchester gallon, which is now known as the US dry gallon.
Both are now referred to as US liquid gallon and US dry gallon, respectively. On the other hand, the Imperial gallon was adopted by the British Empire in 1834.
Thus, A gallon is a common volume measurement unit for measuring liquids and occasionally dry things. 5 gallon is equal to 18.925 L.
Learn more about Liter, refer to the link:
https://brainly.com/question/25546396
#SPJ1
Which pictures show the car at zero acceleration? A. Picture 1 only. B. Pictures 1 and 3. C. Picture 2 and 4. D. Picture 4 only.

Answers
Picture 1 shows choice B
Explanation:
picture 1 shows the car to which the net force is a zero
What color does red cabbage juice make when mixed with a citrus cleaner?
What color does red cabbage juice make when mixed with a dishwasher soap?
(WILL MARK BRAINLIEST!!!)
Answers
Answer:
Explanation:
green
Hydrogen, deuterium, and singly ionized helium are all examples of one-electronatoms. The deuterium nucleus has the same charge as the hydrogen nucleus, and almost exactlytwice the mass. The helium nucleus has twice the charge of the hydrogen nucleus, and almostexactly four times the mass. Make a prediction of the ratios of the ground state energies of theseatoms, considering that current spectroscopy accuracy is extremely good (on the order of107).
Answers
Answer:
0.99986
Explanation:
attached below is the detailed solution to the given problem
Express the ratios of the ground state energies of these atoms
ground state energies ( Ed , Ehe )
= Ed / Ehe = -13.5963 / -13.59815
= 0.99986
