Question 1: What are valence electrons, and how are they related to the way atoms react?
Question 2: Explain the "magic number" of helium and the other elements on the periodic table in terms of their valence electrons. What happens when an element reaches this "magic number?"
Answers
1) Valence electrons are the electrons which are present in the outermost orbital of the atom and it determines the bonding of atoms.
The valence electron in an atom is defined as the number of electrons present in the outermost orbital of the atom. The number of valence electrons present in the outer orbital determines the bonding of an atom. When two atoms are interact with each other, the electrons present in the outer shell come in contact with each other, and this electron determines how an atom will going to react in a chemical reaction. The valence electrons absorb or release the energy in the form of a photon. For example, when sodium atom reacts with chlorine, it forms ionic compound because sodium and chlorine are present in ion forms. They easily lose or gain electrons.
Therefore, valence electrons are the electrons which are present in the outermost orbital of the atom and it determines the bonding of atoms.
To know more about valence electrons
https://brainly.com/question/13691496
#SPJ4
Related Questions
What is atom economy? A. All of these B. The system that determines how much 1 mole of a pure element costs. C. The measure of how much of the reactants end up as products in a chemical reaction. D. The system of exchanging electrons that occurs in atoms.
Answers
Answer:
C
Explanation:
It's the amount of products you get compared to all the reactants you use. It's kind of like economy in that it's profit, and the higher the atom economy the more products/profit you have.
Answer:
C.) The measure of how much of the reactants end up as products in a chemical reaction.
Explanation:
I got it right on founders edtell.
What phase is Nitrogen at 80K

Answers
Answer:
i believe its gas sorry if i am wrong
Explanation:
.Write a balanced reaction for which the following rate relationships are true?
Rate = -1/2 Δ[N2O5 ]/ Δt = 1/4 Δ[NO 2]/ Δt = Δ[O 2]/ Δt
A) 2 N2O5 → 4 NO2 + O2
B) 4 NO2 + O2 → 2 N2O5
C) 2 N2O5 → NO2 + 4 O2
D) 1/4 NO2 + O2 → 1/2 N2O5
E) 1/2 N2O5 → 1/4 NO2 + O2
Answers
The reaction proceeds from NO2 and O2 to N2O5, with the correct stoichiometry. As [NO2] and [O2] increase, [N2O5] would increase at one-half the rate, which fits the rate laws provided.
The correct answer is (B) 4 NO2 + O2 → 2 N2O5.
To explain why, we need to use the rate laws provided. The first rate law tells us that the rate of the reaction is proportional to the negative change in [N2O5] over time. This means that as [N2O5] decreases, the rate of the reaction will increase. The second rate law tells us that the rate of the reaction is proportional to the positive change in [NO2] over time. This means that as [NO2] increases, the rate of the reaction will increase. Finally, the third rate law tells us that the rate of the reaction is proportional to the positive change in [O2] over time. This means that as [O2] increases, the rate of the reaction will increase.
Now, let's consider each answer choice. Answer A suggests that the reaction proceeds from N2O5 to NO2 and O2. However, this would mean that as [N2O5] decreases, [NO2] and [O2] would increase at the same rate, which does not fit the rate laws provided. Answer C suggests that the reaction proceeds from N2O5 to NO2 and O2, but with a different stoichiometry. Again, this does not fit the rate laws provided.
Answer D suggests that the reaction proceeds from NO2 and O2 to N2O5, but with a different stoichiometry. However, this would mean that as [NO2] and [O2] increase, [N2O5] would increase at one-fourth the rate, which does not fit the rate laws provided.
To know more about stoichiometry visit:-
https://brainly.com/question/28780091
#SPJ11
using acetonitrile (ch3cn) and co2 as your only sources of carbons, identify how you could prepare the following compounds:
Answers
Mark as brainlist

The____focuses light the pupil takes in and changes shape too focus.
A.Lens
B.Sclera
C.Eye
D.Retina
Answers
When you are looking at a near object, the lens needs to become more rounded at the central surface in order to focus the light rays. This ability to change focus for close-up objects is called accommodation. ... The ability of the eye to change the shape of its lens and its focus is known as accommodation.
What occurs when the expectations of a scientist change how the results of an experiment are viewed?
conclusion
bias
data
revision
Answers
Answer:
A bias occurs in an experiment when a scientist expects something to happen and lets this influence how the results are viewed. Scientists try to reduce bias whenever they can by doing the experiment many times and keeping careful notes about observations.
Explanation:
helpp!!
What pair of elements is most likely to form a metallic bond?
a.sodium and arsenic
b.lithium and oxygen
c.magnesium and zinc
d. calcium and selenium
who ever answers gets the branliest !
Answers
Answer:
c.magnesium and zinc
Explanation:
you're welcome
t. g. draper. a logarithmic-depth quantum carry-lookahead adder. quantum inf. comput., 6(4):351, 2006
Answers
The study focuses on an effective addition circuit and incorporates carry-lookahead arithmetic approaches.
The work showed an effective addition circuit that used methods from the traditional carry-lookahead arithmetic circuit. Two n-bit values are input into the quantum carry-lookahead (QCLA) adder, which adds them in O(log n) depth with On supplementary qubits. It typically offered a few variants that add modulo 2n and modulo 2n - 1, as well as in-place and out-of-place versions.
The method of choice incorporated in the past has been the ripple-carry addition circuit with linear depth. Our innovation significantly lowers the cost of addiction while just slightly increasing the number of qubits needed. Current modular multiplication circuits can significantly shorten the run-time of Shor's algorithm by utilising the QCLA adder.
Read more about circuit on:
https://brainly.com/question/26064065
#SPJ4
Complete Question:
Explain the study of t. g. draper. a logarithmic-depth quantum carry-lookahead adder. quantum inf. comput., 6(4):351, 2006.
What does a reaction energy diagram represent ?
A. The changes in concentrations with time
B. The changes in energy during reaction reaction
C. The changes in phases with temperature
D.The changes in the reaction rate with time

Answers
Answer:
B. changes in energy during the reaction
Explanation:
y-axis measures "energy" and x-axis measures "reaction progress". Those units fit well with (B) and not so well with concentration and time (A), phases and temperature (C), or reaction rate and time (D).
Answer:
b. changes in energy during reaction
Explanation:
graph
the term rubber refers to any of the natural or synthetic polymers having two main properties: deformation under strain and elastic recovery after vulcanization. what are these polymers called?
Answers
The polymers that exhibit deformation under strain and elastic recovery after vulcanization are commonly referred to as elastomers. Elastomers can be either natural or synthetic and are characterized by their ability to stretch under stress and return to their original shape when the stress is removed.
The term "rubber" encompasses a wide range of materials that possess the characteristic properties of deformation under strain and elastic recovery after vulcanization. These materials are known as elastomers. Elastomers can be found in both natural and synthetic forms. Natural rubber, derived from the latex of certain plants, such as the rubber tree, is an example of a naturally occurring elastomer. Synthetic elastomers, on the other hand, are manufactured through chemical processes and include materials like styrene-butadiene rubber (SBR), neoprene, silicone rubber, and polyurethane, among others. Elastomers owe their unique properties to their molecular structure, which consists of long polymer chains with flexible segments. When a force is applied to an elastomer, the chains undergo significant stretching or deformation. However, unlike other polymers, elastomers have the ability to recover their original shape after the force is removed. This elastic behavior is achieved through a process called vulcanization, where the elastomer is chemically treated to cross-link the polymer chains. The cross-links act as physical bridges between the chains, providing stability and allowing the elastomer to retain its shape even after deformation. The combination of deformation under strain and elastic recovery after vulcanization makes elastomers highly useful in various applications. Their ability to stretch and recoil makes them ideal for products requiring flexibility, resilience, and durability. Elastomers are used extensively in industries such as automotive, aerospace, construction, healthcare, and consumer goods, where they find applications in tires, seals, gaskets, hoses, adhesives, medical devices, and countless other products that benefit from their unique properties
Learn more about styrene-butadiene rubber here: brainly.com/question/29851845
#SPJ11
reading:
The accelerometer keeps track of how quickly the speed of your vehicle is changing. When your car hits another car—or wall or telephone pole or deer—the accelerometer triggers the circuit. The circuit then sends an electrical current through the heating element, which is kind of like the ones in your toaster, except it heats up a whole lot quicker. This ignites the charge which prompts a decomposition reaction that fills the deflated nylon airbag (packed in your steering column, dashboard or car door) at about 200 miles per hour. The whole process takes a mere 1/25 of a second. The bag itself has tiny holes that begin releasing the gas as soon as it’s filled. The goal is for the bag to be deflating by time your head hits it. That way it absorbs the impact, rather than your head bouncing back off the fully inflated airbag and causing you the sort of whiplash that could break your neck. Sometimes a puff of white powder comes out of the bag. That’s cornstarch or talcum powder to keep the bag supple while it’s in storage. (Just like a rubberband that dries out and cracks with age, airbags can do the same thing.) Most airbags today have silicone coatings, which makes this unnecessary. Advanced airbags are multistage devices capable of adjusting inflation speed and pressure according to the size of the occupant requiring protection. Those determinations are made from information provided by seat-position and occupant-mass sensors. The SDM also knows whether a belt or child restraint is in use.
Today, manufacturers want to make sure that what’s occurring is in fact an accident and not, say, an impact with a pothole or a curb. Accidental airbag deployments would, after all, attract trial lawyers in wholesale lots. So if you want to know exactly what the deployment algorithm stored in the SDM is, just do what GM has done: Crash thousands of cars and study thousands of accidents. The Detonation: Decomposition Reactions Manufacturers use different chemical stews to fill their airbags. A solid chemical mix is held in what is basically a small tray within the steering column. When the mechanism is triggered, an electric charge heats up a small filament to ignite the chemicals and—BLAMMO!—a rapid reaction produces a lot of nitrogen gas. Think of it as supersonic Jiffy Pop, with the kernels as the propellant. This type of chemical reaction is called “decomposition”. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. A reaction is also considered to be decomposition even when one or more of the products are still compounds.
Equation 1. general form of decomposition equations When sodium azide (NaN3) decomposes, it generates solid sodium and nitrogen gas, making it a great way to inflate something as the small volume of solid turns into a large volume of gas. The decomposition of sodium azide results in sodium metal which is highly reactive and potentially explosive. For this reason, most airbags also contain potassium nitrate and silicon dioxide which react with sodium metal to convert it to harmless compounds. Equation 2. decomposition of sodium azide Ammonium nitrate (NH4NO3), though most commonly used in fertilizers, could also naturally decompose into gas if it’s heated enough, making it a non-toxic option as an airbag ingredient. Compared to the sodium axide standard, half the amount of solid starting material is required to produce the same three total moles of gas, though that total is comprised of two types, dinitrogen monoxide (N2O) and water vapor (H2O). Equation 3. decomposition of ammonium nitrate Highly explosive compounds like nitroglycerin (C3H5N3O9) are effective in construction, demolition, and mining applications, in part, because the products of decomposition are also environmentally safe and nontoxic. However, they are too shock-sensitive for airbag applications. Even a little bit of friction can cause nitroglycerin to explode, making it difficult to control. The explosive nature of this chemical is attributed to its predictable decomposition which results in nearly five times the number of moles of gas from only four moles of liquid starting material when compared to both sodium azide and ammonium nitrate alternatives.
You're are NOT answering this: Scientific question: How does the choice of chemical ingredient ia airbn ag influence their effectiveness.
As you talks about the dimensional analysis setup, stock and explain each part using da ts format he article.
Point directly to the collected data as evidence. Since the scientific question relates the chemical ingredients to effectiveness, you might consider discussing all the outcomes for each chemical ingredient (time, volume, popped/not inflated, enough/inflated perfectly, amount initially used separately.
Answers
The choice of chemical ingredients in airbags influences their effectiveness in several ways:
Time: Sodium azide ignites faster than ammonium nitrate, decomposing in under 1/25 sec vs. requiring heating to ignite. Sodium azide's faster ignition leads to quicker airbag inflation.
Volume: Sodium azide produces 3 moles of gas from 2 moles of solid, while ammonium nitrate produces 3 moles of gas from 4 moles of solid to achieve the same total moles of gas. Less starting material is needed for sodium azide to produce the same volume of gas.
Amount used: To produce the same volume of gas, half the amount of solid sodium azide (2 moles) is required compared to ammonium nitrate (4 moles). Less ingredient is needed for sodium azide.
Popped/Not inflated: Highly explosive compounds like nitroglycerin are too shock-sensitive and difficult to control, easily popping the airbag before it fully inflates. Sodium azide and ammonium nitrate can be controlled to properly inflate the airbag.
Enough/Inflated perfectly: Advanced airbags with sensors can determine the optimal amount of each chemical to inflate based on occupant size. Multiple stages of inflation are possible for perfect inflation control and cushioning. Single-stage less controlled explosions may over- or under-inflate the airbag.
Data:
Equation 1: General decomposition equation
Equation 2: Decomposition of sodium azide
Equation 3: Decomposition of ammonium nitrate
Sodium azide → 3 moles gas / 2 moles solid
Ammonium nitrate → 3 moles gas / 4 moles solid
Nitroglycerin → 5 moles gas / 4 moles liquid
In summary, the choice of chemical impacts how quickly, how much, and how controllably the airbag inflates. Sodium azide and ammonium nitrate can be optimized and controlled, while nitroglycerin is too volatile. Less material is needed for faster-acting sodium azide. Advanced sensors enable perfectly inflating multistage airbags regardless of the chemical mixture. The data and equations show the mole ratios for each chemical decomposition.
Please let me know if you need any clarification or have additional questions!
What mass (g) of NaN3 is required to provide 40.0 L of N2 at 25.0°C and 763 torr?
Answers
The needed mass of NaN3 is 71.175 grammes.
How much NaN3 must be produced in grammes?As seen above, for every 2 moles of sodium azide that break down, 3 moles of nitrogen gas are created. This mole ratio will be helpful in figuring out how much NaN3 N a N 3 is required to make 10.0 cubic feet of N2 gas. Hence, to generate 10.0 cubic feet of N2 gas, 547 grammes of NaN3 are required.
What gas makes up the majority of an air bag that has been inflated by the NaN3 reaction?The sodium azide, or NaN3, chemical would hold the key to the solution. Nitrogen gas, which may instantly inflate an airbag, is released when this chemical is ignited by a spark.
To know more about mass visit:-
https://brainly.com/question/13197537
#SPJ9
The _____ protects Earth from meteoroids and other objects that may fall from space.
Answers
Mesophere (I'm pretty sure that's right)
What are the compounds that you can find in mayonnaise
Answers
HEY!
Mayonnaise is an oil in water (O/W) emulsion; ingre- dients are primarily vegetable oil, egg yolk, sodium chloride, water and vinegar. Its relative stability towards microbial spoil- age has been attributed to the high salt content (in the water phase) and low pH, due to the vinegar.
thank me later
carryonlearing
The yield of a chemical process is being studied. From previous experience, yield is known to be normally distributed and σ=3 . The past five days of plant operation have resulted in the following percent yields: 91.6, 88.75, 90.8, 89.95, and 91.3.
a. Find a 95% two-sided confidence interval on the true mean yield. b. Find a 95% upper level confidence interval on the true mean yield. c. How many samples of yield is needed to obtain a 95% two-sided confidence interval with width 1? d. If we don't know the value of σ , how would the 95% two-sided confidence interval be?
Answers
The two-sided confidence interval is (87.85 ≤ μ ≤ 93.11) , with 87.85 being the lower level and 93.11 being the upper level.
What is a yield in an experiment?The amount of product you actually get after doing an experiment is known as the experimental yield. Calculating the % yield will show you how much of the theoretical yield you actually achieved in a particular experiment. The quantity of pure and dry product produced in a chemical reaction is known as the reaction yield (absolute yield). The relative or percentage yield (%) is typically determined in order to assess the effectiveness of a chemical process in organic synthesis.
How is the yield of a chemical reaction determined?The percent yield can be calculated by applying the following formula:%yield = (actual yield/theoretical yield) x 100.
Brifieng:Step-by-step explanation:
Given the data:
91.6, 88.75, 90.8, 89.95, 91.3
Mean, m = Σx / n
n = sample size = 5
Mean = 452.4 / 5 = 90.48
Standard deviation, σ = 3
Zcritical at 95% = 1.96
Confidence interval :
Mean ± Error margin
Error margin = Zcritical*σ/sqrt
Error margin = 1.96 * 3/sqrt(5)
Error margin = 2.630
Lower boundary : 90.48 - 2.630 = 87.85
Upper boundary : 90.48 + 2.630 = 93.11
(87.85 ; 93.11)
To know more about Yield of a Chemical visit:
https://brainly.com/question/14408642
#SPJ4
37L of carbon dioxide decomposes. what mass of carbon results?
PLEASE HELP <3
Answers
The mass of carbon that resulted from the decomposition of 37 L of carbon dioxide is 19.8 g
Balanced equationCO₂ —> C + O₂
From the balanced equation above,
1 L of CO₂ decomposed to produce 1 L of C.
Therefore,
37 L of CO₂ will also decompose to produce 37 L of C
How to determine mass of CarbonAt standard temperature and pressure (STP),
22.4 L = 1 mole of C
Therefore,
37 L = 37 / 22.4
37 L = 1.65 mole of C
Thus, the mass of Carbon can be obtained as follow:
Mole of C = 1.65 mole Molar mass of C = 12 g/mol Mass of C =?Mass = mole × molar mass
Mass of C = 1.65 × 12
Mass of C = 19.8 g
Thus, 19.8 g of carbon were obtained from the reaction.
Learn more about stoichiometry:
https://brainly.com/question/1473580
At what point is the electric field around the positive charge the weakest?

Answers
Answer:
A
Explanation:
as u move away from static charge the electric field intensity decreses
How many electrons must you add to Boron-10 to create a neutral atom? How many electrons must you add to Boron-11 to create a neutral atom? Why?
Answers
Answer:
What make Boron Boron is that it has 5 protons, and will therefor have 5 electrons in the unionized state. While I was looking this up I learned that there are two stable isotopes, Boron 10 with five neutrons, and boron 11 with six. The more common is boron 11, which is 80.1% of naturally occuring boron.
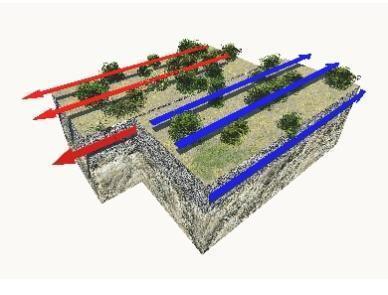
Study the image.
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
Calculate the molar mass, in g/mol, for the compound strontium nitrate, Sr(NO3)2.
Answers
By definition, the molar mass for the compound strontium nitrate Sr(NO₃)₂ is 211.62 g/mole.
Definitio of molar massThe molar mass of substance is a property defined as its mass per unit quantity of substance, in other words, molar mass is the amount of mass that a substance contains in one mole.
The molar mass of a compound (also called Mass or Molecular Weight) is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of strontium nirateIn this case, you know the molar mass of the elements is:
Sr= 87.62 g/moleN= 14 g/moleO= 16 g/moleSo, the molar mass of the compound Sr(NO₃)₂ is calculated as:
Sr(NO₃)₂= 87.62 g/mole + 2× (14 g/mole + 3×16 g/mole)
Solving:
Sr(NO₃)₂= 211.62 g/mole
Finally, the molar mass for the compound strontium nitrate Sr(NO₃)₂ is 211.62 g/mole.
Learn more about molar mass:
https://brainly.com/question/4355575?referrer=searchResults
https://brainly.com/question/2166483?referrer=searchResults
What dicarbonyl compound is needed to prepare the following compound by an intramolecular aldol reaction?.
Answers
The given compound has a cyclic structure, indicating that it was formed by an intramolecular aldol reaction. The reactant in this reaction would be a dicarbonyl compound.
One possible dicarbonyl compound that could be used in this reaction is 3-oxo heptane dioic acid, also known as beta-ketoglutaric acid. This compound has a cyclic structure with two carbonyl groups that can undergo aldol condensation and cyclization to form a six-membered ring. The resulting product would have a similar structure to the given compound.
To know more about compound visit :
https://brainly.com/question/13516179
#SPJ11
if you weigh out 0.205 g of salicylic acid, what is the theoretical yield for aspirin?
A. 0.157 g
B. 0.267 g
C. 0.134 g
D. 0.0786 g
E. none of the above choices
Answers
I can’t figure out if I’m doing this right or not

Answers
A structural isomer is a compound that has the same number of atoms but is arranged differently. Therefore, to know which will be the structural isomer of the compound they give us, we must count the atoms of each compound.
When comparing the compounds we see that pent-2-yne has C5H8 just like 3-methylcyclobutene, therefore these two compounds are structural isomers.
The answer will be: 3-methylcyclobutene



Secondary succession can only occur as a transition from primary succession.
Please select the best answer from the choices provided
T
F
Answers
Answer:
T
Explanation:
Hope this helps(Truth)
:)
Answer:
The Answer Is False!
Explanation:
Your Welcome, I did the test.
The following is a sample question for brainliest. Brainliest will be given to the person who can answer the following question with the most details and accuracy. I do have sample answers that I wrote to compare yours too, though answers obviously may vary.
What is Chemistry? (At least one paragraph.)
Answers
Answer:
Chemistry is the branch of science that deals with the properties, composition, and structure of elements and compounds, how they can change, and the energy that is released or absorbed when they change. Traditionally, chemistry has been broken into five main subdisciplines: Organic, Analytical, Physical, Inorganic and Biochemistry. Chemistry is involved in everything we do. The reason why chemistry touches everything we do is because almost everything in existence can be broken down into chemical building blocks.
Explanation:
Hope this helps
A Rectangular Prism has a height of 4cm, a width of 5cm, a length of 1.2 cm, and a mass of 85,000 g. What is it's density?
A. .00000035416 g/cm3
B. 00035416 g/cm^3
C. 354.16 g/cm^3
D. 3541.6 g/cm
Answers
Answer:
The answer is D.
Explanation:
Volume (V) = l x w x h
V = 4 x 5 x 1.2
V = 20 x 1.2
V = 24
Density = Mass/Volume or D = M/V
D = 85,000/24
D = 3541.66666666
when magnesium is burned in the presence of oxygen, it produces magnesium oxide according to the following chemical equation. if 3.45 grams of mg are burned, how many grams of mgo are produced?
Answers
if 3.45 grams of magnesium are burned, 1.725 grams of magnesium oxide are produced. The chemical equation for the reaction of magnesium with oxygen is 2MgO.
How to calculate mgo are produced?Magnesium is a chemical element that is highly reactive, meaning that it easily reacts with other substances. When magnesium is burned in the presence of oxygen, it reacts with the oxygen to form magnesium oxide,
The chemical equation for the reaction of magnesium with oxygen is:
2Mg + O2 -> 2MgO
This equation tells us that for every 2 grams of magnesium that react with oxygen, 2 grams of magnesium oxide are produced. Therefore, if 3.45 grams of magnesium are burned, the number of grams of magnesium oxide produced can be calculated as:
3.45 grams Mg / 2 grams Mg/1 gram MgO = 1.725 grams MgO
Therefore, if 3.45 grams of magnesium are burned, 1.725 grams of magnesium oxide are produced.
To learn more about magnesium refer :
https://brainly.com/question/25860912
#SPJ1
compare LPG and CNG as a fuel with coal
Answers
How is it possible to change the shape of your molecular models without breaking any of the covalent bonds?
Answers
Answer:
A molecule's shape strongly affects its physical properties and the way it interacts with other molecules, and plays an important role in the way that biological molecules (proteins, enzymes, DNA, etc.) interact with each other.
Explanation:
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, by rotation of molecule or bond , we can change the shape of molecule.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
The compound that is ionic in nature can be dissociated very easily in water. Since ionic compounds are polar in nature, they readily dissolve in water. To change the shape of your molecular models without breaking any of the covalent bonds is by rotating the molecule or a bond.
Therefore, by rotation of molecule or bond , we can change the shape of molecule.
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ2
a student was synthesizing aspirin in the laboratory. using the amount of limiting reactant, she calculated the mass of aspirin that should form as 11.74 g. when she weighed her aspirin product on the balance, its mass was 2.63 g. calculate the percent yield for this synthesis.
Answers
If the student was synthesizing aspirin in the lab and she calculated the mass of aspirin that should form as 11.74 g and when she weighed her aspirin product on the balance which was 2.63 g. The percent yield can be 22.4 %.
The amount of a product received from a reaction is indicated by the percent yield.
The theoretical yield is given, which is 11.74 g and the actual yield is given which is 2.63 g.
Now, we use the formula of the percentage (%) yield,
percentage (%) yield = \((\frac{ Actual yield }{ Theoretical yield } )\) × 100
% yield = \((\frac{2.63}{11.74})\) × 100
% yield = 22.4 %
So, the percentage (%) yield is 22.4 %.
To learn more about Percent yield here :
https://brainly.com/question/13711435?referrer=searchResults
#SPJ4