Question 2:heat (5 points) a. Describe the following heat equations and identify the indicated variables. (3 points) I. Q= mct; identify c. (1 point) ii. Q=ml vapor; identify l vapor (1 point) iii. Q= ml fusion; identify l fusion (1 point)
Answers
I. Q = mct; c is the specific heat capacity, II. Q = ml vapor; l vapor is the latent heat of vaporization,III. Q = ml fusion; l fusion is the latent heat of fusion.
What is vaporization?Vaporization is the process of a substance changing from its liquid form to its gaseous form. It occurs when the substance absorbs heat, causing its molecules to move faster and further apart, converting it from a liquid to a gas. Vaporization is a process that occurs when a liquid is heated to its boiling point and then cooled, causing the molecules to break apart and form a vapor. Vaporization can also occur when a solid is heated until it sublimates, or when the molecules of the solid are broken down into a gas. Vaporization is an important part of the water cycle, and it is also used in many industries, such as chemical production, pharmaceutical manufacturing, and food processing.
To learn more about vaporization
https://brainly.com/question/26306578
#SPJ4
Related Questions
Gandhiji came back to india _____
a) 1915
b) 1885
c) 1526
d) 1877
Answers
I hope this helps :)
Answer:
January 9th 1915
Explanation:
Match the K’eq values with the appropriate delta G°’ values. Define each as exergonic, endergonic, or at equilibrium.
K'eq a. 1 b. 10^-5 c. 10^4 d. 10² e. 10^-1 AG°' (kJ/mol)
i. 28.53
ii. -11.42
iii. 5.69 iv. 0
v. -22.84
Answers
Exergonic: Reactions with negative delta G°’ values are exergonic reactions that release free energy.Exergonic means that the reaction will occur spontaneously. The value of delta G°’ is less than zero.
Endergonic: When the delta G°’ value is positive, the reaction is endergonic. These are reactions that need to be powered by an external force to occur.
At equilibrium: When delta G°’ = 0, the reaction is at equilibrium. The forward and reverse reactions occur at the same rate, and the concentrations of reactants and products do not change. Thus, the reaction is at equilibrium.
Here is how the K’eq values should be matched with the appropriate delta G°’ values:
K'eq a. 1 : 0kJ/mol b. 10⁻⁵ : 22.84 kJ/mol c. 10⁴ : -28.53 kJ/mol d. 10² : -5.69 kJ/mol e. 10⁻¹ : 11.42 kJ/mol AG°' (kJ/mol)
Therefore, the correct match for K’eq values with the appropriate delta G°’ values are as follows:
K'eq = 10⁴ corresponds to delta G°’ = -28.53 kJ/mol, which is exergonic.
K'eq = 10⁻⁵ corresponds to delta G°’ = 22.84 kJ/mol, which is endergonic.
K'eq = 10² corresponds to delta G°’ = -5.69 kJ/mol, which is exergonic.
K'eq = 1 corresponds to delta G°’ = 0 kJ/mol, which is at equilibrium.
K'eq = 10⁻¹ corresponds to delta G°’ = 11.42 kJ/mol, which is endergonic.
For more questions on exergonic and endergonic reactions: https://brainly.com/question/1560981
#SPJ11
How many atoms are present in 2.30 moles of Carbon?
3.82 x 10-24 atoms -a
3.82 x 1023 atoms-b
1.38 x 1024 atoms-c
6.02 x 1023 atoms-d
Answers
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.20 M KOH(aq). A) red B) yellow C) red-yellow mixture D) orange E) The indicator keeps its original color.
Answers
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0.
In a 0.20 M KOH(aq) solution, KOH dissociates to form hydroxide ions (OH⁻) in water.
The hydroxide ions can react with water to produce hydroxide ions and hydroxide ions can increase the concentration of hydroxide ions in the solution, resulting in a basic pH.
Since KOH is a strong base, it completely dissociates in water, leading to a high concentration of hydroxide ions. The presence of a high concentration of hydroxide ions indicates a basic solution.
Based on the given pH range for phenol red, which changes from yellow to red between pH 6.6 and 8.0, we can infer that in a 0.20 M KOH(aq) solution, the indicator will assume a RED color.
Therefore, the correct answer is A) red.
what challenges arise when nuclear material is removed from a power plant?
Answers
Answer:
it destroy the surrounding area
2H2O --> 2H2 + O2 If you begin with 20 grams of H2O, how many moles of O2 are produced?
Answers
Answer:
17.78 gm
Explanation:
2 H20 mole weight = 36 gm
which leads to 32 gm O2
36 / 32 = 20 / x x = 17.78 gm
a 10 kg computer accelerates at a rate of 5 m/s2. how much force was applied to the computer?
Answers
The force applied to the 10 kg computer was 50 Newtons.
What is computer ?An electrical device with the capability to accept, store, process, and output data is known as a computer.
The following formula can be used to determine the force exerted on a 10 kilogram computer that is accelerating at a rate of 5 m/s2:
Force = mass x acceleration
Where
mass = 10 kg (given)acceleration = 5 m/s² (given)Plugging in these values, we get:
Force = 10 kg x 5 m/s²
Force = 50 N
Therefore, the force applied to the 10 kg computer was 50 Newtons.
Learn more about computer here : brainly.com/question/21474169
#SPJ1
list the acids present in a car battery
Answers
Answer:
sulphuric acid
Explanation:
What am I? 2. I have 7 valence electrons and have 2 more protons than "phosphorus. What am I? 3. I am in Period 5 and have properties similar to my favorite cousin, gold. What am I? 4. I am the only element with no neutrons in my nucleus. What am I? 5. I am the only element in Period 3 that has 8 electrons in my outermost energy level. What am I? 6. I am the most massive element on the Periodic Table to have electrons in only one energy level. What am I? 7 I have the smallest atomic number of all the elements that have electrons in three different energy levels? What am I?
Answers
Answer:
2. chlorine
3. Copper??
4. Isotope
5. sodium, magnesium, aluminum, silicon, phosphorus, sulfur, or argon
6. Oganesson
7. Hydrogen
Hope these are right!
HELP PLEASE
Which of the following BEST describes
the size of asteroids?
A. All of them are smaller than a kilometer in diameter.
B. All of them are larger than 100 kilometers in diameter.
C. They range from smaller than 1 km to about 300 km in
diameter.
D. They range from 1 km to about 100 km in diameter.
I put chemistry because there’s no astronomy
Answers
Answer:
I think B is the answer so hope
Answer:
It's C
Explanation:
What is the mass of 5.36 mol of ammonia
vapor (NH3)?
Answer in units of g.
Answers
Answer:
91.12 gram
Explanation:
Number of moles of Ammonia = 5.36
Molecular Mass of Ammonia = 14 + 3 × 1 = 17 gram/mol
Mass = Number of moles × Molecular mass
Mass = 5.36 mol × 17 gram/mol = 91.12 gram
CAN SOMEONE OR SOMEBODY HELP ME ASAP



Answers
Answer: ahem...
Explanation: 1. True 2. reproducing 3. All of them 4. i would say true, the only thing is plants dont really digest food and it says "all" organisms... 5. B, C, and D...A is more of defense and not really "maintaining structure"
an 80.0 g sample of metal, initially at 96.0 °c, is placed into 150.0 g of water initially at 26.0 °c in a calorimeter. the final temperature of the water is 28.1 °c. what is the identity of the metal? (the specific heat of water is 4.18 j/g・°c.
Answers
If the final temperature of the water is 28.1 °c, then the identity of metal is Ag.
What is Ag metal?
With the atomic number 47 and the symbol Ag (from the Latin argentum, which is derived from the Proto-Indo-European h2er: "bright" or "white"), silver is a chemical element. It is the highest electrical, thermal, and reflectivity of all metals, and it is a soft, white, lustrous transition metal.
The Latin argentum and Sanskrit argunas, both meaning "bright," are where the word "Ag" originates. Even in the Stone Age, silver was used. Silver use dates back at least 5000 years, according to archeological findings.
To learn more about Ag metal click the given link
https://brainly.com/question/1769963
#SPJ4
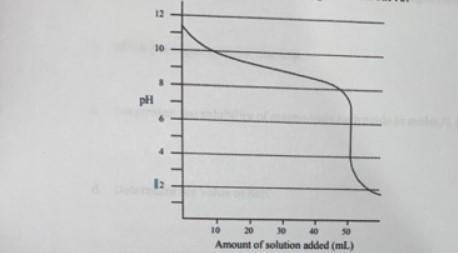
what is the ph of the solution at the equivalence point?
A. 11.3
B. 10.0
C. 9.3
D. 5.3
Answers
The ph of the solution at the equivalence point is 5.3. Option D is correct.
Acid-base titrations are an important analytical technique used to determine the concentration of an acid or base in a solution. In a typical acid-base titration, a solution of known concentration, called the titrant, is slowly added to a solution of unknown concentration, called the analyte, until the reaction between the two is complete.
In some cases, the pH at the equivalence point may be different from 7, depending on the strength of the acid and base being used in the titration. For example, if a weak acid is being titrated with a strong base, the equivalence point will be greater than pH 7. Conversely, if a strong acid is being titrated with a weak base, the equivalence point will be less than pH 7.
However, if the question is asking for the pH at the endpoint of the titration, rather than the equivalence point, the pH will depend on the specific indicator being used in the titration. At the endpoint, the solution will be slightly basic if a strong base is used, and the pH will be greater than 7. The exact pH at the endpoint will depend on the amount of excess base present in the solution and the specific indicator used in the titration. Option D is correct.
To know more about the Equivalence point, here
https://brainly.com/question/29999744
#SPJ4
The half-life of Radon-222 is 3.8 days. If a 10 gram sample is present, how many days will it take to have less than one gram remaining?
Answers
(alt + F4) might be the answer you are looking
Explanation:
I'm not exactly sure how I came up with this answer but it is for sure the answer
A properly designed experiments changes one variable at a time. A student wanted to know which type of sugar would yield the greatest amount of energy for the cell when metabolized during cellular respiration by mitochondria. The student mixed yeasts in three different sugar solutions: maltose, glucose, sucrose. The three sets of yeasts were monitored to determine which sugar type yielded the most energy. List three factors that the student must keep constant during the experiment
Answers
Answer:
The amount of water
The amount of sugar and
The amount of yeast
Explanation:
A constant variable is that variable in an experiment which must be kept the same for all groups in order not to alter the results of the experiment.
In this question, the independent variable i.e. the variable being manipulated is the TYPE OF SUGAR USED while the dependent variable i.e. variable that responds to change is AMOUNT OF ENERGY. Three different types of sugar were used viz: maltose, glucose, and sucrose. To not alter the outcome of the experiment, the constants of this experiment i.e. variables that must be the same for all groups of the experiment, include the following:
- The amount of water
- The amount of sugar
- The amount of yeast used
Describe how you would prepare a pure dry sample of lead(II) sulfate crystals starting from solutions of lead(II) nitrate and sodium sulfate.
Include a series of key steps in your answer.
Answers
Answer:
Method: Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker. Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod.
what happens to the atomic size of elements on moving from left to right in a period of modern periodic table?
Answers
Answer: the atomic size will decrease
Explanation:

Answer:
It will remain the same because it is moving up and down to and fro it can not increase nor decrease or else will become an ion
Find the quantinum numbers n,m,l,s for the last of potassium layer pleasee help explain correctly all
Answers
Answer:
Quantum numbers of the outermost electron in potassium:
\(n = 4\).\(l = 1\).\(m_l = 0\).Either \(m_s = 1/2\).Explanation:
Refer to the electron configuration of a potassium atom. The outermost electron in a ground-state potassium atom is in the \(4s\) orbital (fourth \(s\) orbital.)
The quantum number \(n\) (the principal quantum number) specifies the main energy shell of an electron. This electron is in the fourth main energy shell (as seen in the number four in the orbital.) Hence, \(n = 4\) for this electron.
The quantum number \(l\) (the angular momentum quantum number) specifies the shape (\(s\), \(p\), \(d\), etc.) of an electron. \(l = 1\) for \(s\!\) orbitals (such as the one that contains this electron.
Quantum numbers \(n\) and \(l\) specify the shape of an orbital. On the other hand, the magnetic quantum number \(m_l\) specifies the orientation of these orbitals in space.
However, \(s\) orbitals are spherical. Regardless of the value of \(n\), the only possible \(m_l\) value for electrons in \(s\!\) orbitals is \(m_l = 0\).
The spin quantum number \(m_s\) distinguishes between the two electrons in an orbital. The two possible values of \(m_s \!\) are \((+1/2)\) and \((-1/2)\). Typically, the first electron in an orbital is assigned an upward (\(\uparrow\)) spin, which corresponds to \(m_s = (+1/2)\).
Animals use oxygen in the process of
blank
and emit CO2 as a byproduct.
Answers
Answer:
Respiration
Explanation:
The act of breathing, simple as that!
Hope this helped!!
please help me. :)))

Answers
46.12 grams of water are produced when 35 grams of \(C_6H_1_0\) react with 45 grams of \(O_2\).
Stoichiometric problemThe balanced equation for the combustion of C6H10 (cyclohexene) with O2 is:
\(C_6H_{10} + O_2 - > CO_2 + H_2O\)
From the balanced equation, we can see that 1 mole of \(C_6H_1_0\) reacts with 6 moles of O2 to produce 6 moles of water.
First, let's calculate the number of moles of C6H10 and O2:
Molar mass of C6H10 = 6(12.01 g/mol) + 10(1.01 g/mol) = 82.16 g/mol
Number of moles of C6H10 = mass / molar mass = 35 g / 82.16 g/mol ≈ 0.426 mol
Molar mass of O2 = 2(16.00 g/mol) = 32.00 g/mol
Number of moles of O2 = mass / molar mass = 45 g / 32.00 g/mol ≈ 1.406 mol
From the stoichiometry of the balanced equation, we can determine that 0.426 moles of C6H10 will produce 0.426 * 6 = 2.556 moles of water.
Now, let's calculate the mass of water produced:
Molar mass of H2O = 2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
Mass of water = number of moles of water * molar mass of water
= 2.556 mol * 18.02 g/mol
≈ 46.12 g
Therefore, approximately 46.12 grams of water are produced when 35 grams of C6H10 reacts with 45 grams of O2.
More on stoichiometric problems can be found here: https://brainly.com/question/32305503
#SPJ1
The diagram below shows two substances. What name is given to bond X?

Answers
Answer:
single covalent bond
Explanation:
The bond formed between two atoms each sharing one electron is called single covalent bond
The figure 1 is the structure of diamond. The type of bond in diamond is covalent sp³ bond. DIamond exists in hexagonal arrangement of carbon atoms.
What is allotrope?Allotropes are substances formed from a single elements with different number in various size and shape. Carbon exhibit catenation property which means it can forms chains with other carbon in any large number.
The allotropes of carbon are diamond, graphene and fullerene. Diamond is hexagonal arrangement of carbons atom bonded covalently in sp³ hybridisation.
In graphene as shown in the second figure, bonded carbons atoms are arranged as layers where carbon is in sp²hybridisation. In the case of fullerenes, there are both hexagonal and pentagonal rings adjacent to each and are formed by 60-70 carbons.
In all these allotropes, the bond type between two carbons atoms is covalent. Covalent bond is formed by sharing of electrons between the two atoms.
To find more about diamond, refer the link below:
https://brainly.com/question/1120149
#SPJ2
The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride. This polymer will form Choose... To remove the nylon, Choose... Choose... in the 5% hexamethylenediamine in the 5% adipoyl chloride in between layers of the solutions
Answers
The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride. This polymer will form between layers of the solutions. To remove the nylon, one can choose to dissolve it in the 5% hexamethylenediamine or in the 5% adipoyl chloride.
Nylon, a synthetic polymer, is produced from the combination of adipoyl chloride and hexamethylenediamine. This process is called the synthesis of nylon. Nylon is a highly flexible material that is resistant to wear and tear, as well as chemical and heat degradation. The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride, respectively, for the two reactants to be mixed together.
The reaction between these two chemicals is exothermic, which means that it releases heat. The heat generated in the reaction drives the reaction forward, resulting in the formation of nylon. The chemical formula for nylon is (-CO-NH-)n, where n is a large number that reflects the degree of polymerization. To remove the nylon, it is soaked in an acid solution. The acid dissolves the nylon, separating it into its constituent components, which can then be purified and reused.
The most commonly used acid for this process is hydrochloric acid. The process of removing nylon from its solvent is called the "acid-catalyzed hydrolysis of nylon." Nylon is used in a variety of applications, including textiles, packaging materials, and electrical components, among others. Its properties make it ideal for use in applications that require durability, strength, and flexibility. Nylon's physical properties, including its resistance to heat and chemical degradation, make it ideal for use in applications such as electrical insulation, packaging materials, and textiles.
Know more about nylon here:
https://brainly.com/question/25835424
#SPJ8
what did henry moseley contribute to the periodic table?
Answers
Answer:
In 1913 he used self-built equipment to prove that every element's identity is uniquely determined by the number of protons it has. His discovery revealed the true basis of the periodic table and enabled Moseley to predict confidently the existence of four new chemical elements, all of which were found.
The ksp of yttrium fluoride, yf3, is 8. 62 × 10-21. Calculate the molar solubility of this compound
Answers
The molar solubility of yttrium fluoride (YF3) is 3.46 × 10-6 M.
The ksp of yttrium fluoride is 8.62 × 10-21. The molar solubility of this compound can be determined using the following formula:Ksp = [Y3+][F-]3We can set the molar solubility of yttrium fluoride as 'x'.
This is because the solubility of the yttrium fluoride will lead to the concentration of yttrium ions and fluoride ions. The Ksp expression for yttrium fluoride can be represented as follows:
Ksp = (x)(3x)3 = 27x4
where '3x' is the molar solubility of F-.
We can substitute Ksp value in the above expression and then solve for x:
8.62 × 10-21 = 27x4x = 3.46 × 10-6 M
Thus, the molar solubility of yttrium fluoride is 3.46 × 10-6 M.
To conclude, the molar solubility of yttrium fluoride (YF3) is 3.46 × 10-6 M.
To know more about molar solubility VISIT:
https://brainly.com/question/31043999
#SPJ11
What would happen on the Earth if the Sun’s radiation could not travel through space?
Answers
Answer:
We'd all freaking freeze to death and be standing there like the black beetles trend.
Explanation:
This is a joke, please don't take this seriously.
write balanced equations for each of the following by insert- ing the correct coefficients in the blanks: (a) cu(no3)2(aq) koh(aq) ⟶ cu(oh)2(s) kno3(aq) (b) bc13(g) h2o(l) ⟶ h3(bo3(s) hc1(g) (c) casio3(s) hf(g) ⟶ sif4(g) caf2(s) h2o(l) (d) (cn)2(g) h2o(l) ⟶ h2c2o4(aq) nh3(g
Answers
The balanced reaction equations are given below. They are all examples of double displacement.
a) balanced reaction equation of the reaction between aqueous copper(II) nitrate and aqueous potassium hydroxide, resulting in the formation of solid copper(II) hydroxide and aqueous potassium nitrate
\(Cu(NO_{3} )_{2}(aq)+2KOH(aq)\) → \(Cu(OH)_{2} (s) + 2KNO_{3}(aq)\)
b) balanced reaction equation of the double displacement between gaseous boron cyanide and liquid water resulting in the formation of solid boric acid and gaseous hydrogen cyanide
\(2B(CN)_{3} (g) + 6H_{2} O\) → \(2H_{3} BO_{3} (s)+6HCN(g)\)
c) balanced reaction equation between solid calcium silicate and gaseous hydrogen fluoride resulting in the formation of gaseous silicon fluoride, solid calcium fluoride and liquid water
\(CaSiO_{3}(s) + 6HF (g)\) → \(SiF_{4}(g)+CaF_{2}(s)+3H_{2}O(l)\)
d) balanced reaction equation between cyanogen gas and liquid water resulting in formation of aqueous oxalic acid and gaseous ammonia
\((CN)_{2}(g) + 4H_{2} O (l)\) → \(H_{2} C_{2} O_{4} (aq) +2NH_{3} (g)\)
You can learn more about double displacement reactions here:
brainly.com/question/13522228
#SPJ4
Question 5 of 5
Which two pairs of values can be used to determine the velocity of an object?
O A. Distance and mass
B. Mass and acceleration
O C. Speed and direction
O D. Displacement and time
SUBV

Answers
Answer is D & C
Explanation:
It is not just C or D you need to select TWO options.
Speed and direction, Displacement and time can be used to determine the velocity of an object.
what is velocity ?Velocity is the rate of change in the position of the object with respect to time, it is basically speeding the object in a specific direction.
Velocity is a vector quantity, which shows both magnitude and direction where The SI unit is meter per second (ms-1).
the change in magnitude or the direction of velocity of a body, can lead to acceleration.
The final velocity can be calculated as the simple but few calculations and basic conceptual knowledge are needed.
For more details regarding velocity, visit
brainly.com/question/12109673
#SPJ2
The K a of propanoic acid ( C 2 H 5 COOH ) is 1. 34 × 10 − 5. Calculate the pH of the solution and the concentrations of C 2 H 5 COOH and C 2 H 5 COO − in a 0. 597 M propanoic acid solution at equilibrium
Answers
The pH of the solution is 2.55 and the concentrations of C₂H₅COOH and C₂H₅COO⁻ are 0.594 M and 0.00282 M respectively.
The following chemical equation describes how propanoic acid, a weak acid, will ionise in water.
C₂H₅COOH(aq) + H₂O(l) ↔ C₂H₅COO⁻(aq) + H₃O⁺(aq)
The concentration of the initial solution of propanoic acid is 0.597 M.
The Ka of propanoic acid is 1.34 × 10⁻⁵.
Let the concentration of each of the products is x.
Now, We have Ka = [C₂H₅COO⁻] [H₃O⁺] / [C₂H₅COOH] [H₂O]
or, 1.34 × 10⁻⁵ = x × x / 0.597
or, x² = 0.8 × 10⁻⁵
or, x = 2.82 × 10⁻³ M
or, x = 0.00282 M
So, concentration of C₂H₅COO⁻ = x = 0.00282 M
Concentration of C₂H₅COOH = 0.597 - 0.00282 = 0.594 M
Now, pH of solution = -log[H₃O⁺] = -log[x] = -log(0.00282) = 2.55
To know more about propanoic acid from the link
brainly.com/question/20318658
#SPJ4
determine the volume in liters of metal sample weighting 352.2g and has a density of 7.10gmL.
Answers
Answer:
V = 0.0496 L
Explanation:
Given that,
The mass of a sample, m = 352.2 g
The density pf sample, d = 7.10 g/mL
We need to find the volume of the sample. We know that the density of an object is given by :
\(d=\dfrac{m}{V}\\\\V=\dfrac{m}{d}\\\\V=\dfrac{352.2}{7.1}\\\\V=49.6\ mL\)
or
V = 0.0496 L
So, the volume of the sample is 0.0496 L.