silver nitrate and iron (III) chloride are reacted. 27.0 g silver nitrate and 43.5 g iron (III) chloride are used in the reaction.
3 AgNO3 + FeCl3 --> 3 AgCl + Fe(NO3)3
1. Determine the limiting reactant for this reaction.
Answers
Answer:
To determine the limiting reactant, we need to calculate the amount of product that can be formed from each reactant and see which reactant produces the least amount of product. We will use the balanced chemical equation:
3 AgNO3 + FeCl3 --> 3 AgCl + Fe(NO3)3
First, let's calculate the amount of product that can be formed from the silver nitrate:
From the balanced equation, we know that 3 moles of silver nitrate react with 1 mole of iron (III) chloride to produce 3 moles of silver chloride and 1 mole of iron (III) nitrate.
Molar mass of AgNO3 = 107.87 g/mol
Molar mass of AgCl = 143.32 g/mol
Using the given mass of silver nitrate:
27.0 g AgNO3 x (1 mol AgNO3 / 107.87 g AgNO3) x (3 mol AgCl / 3 mol AgNO3) x (143.32 g AgCl / 1 mol AgCl) = 92.9 g AgCl
Next, let's calculate the amount of product that can be formed from the iron (III) chloride:
Molar mass of FeCl3 = 162.20 g/mol
Molar mass of Fe(NO3)3 = 241.81 g/mol
Using the given mass of iron (III) chloride:
43.5 g FeCl3 x (1 mol FeCl3 / 162.20 g FeCl3) x (3 mol AgCl / 1 mol FeCl3) x (143.32 g AgCl / 1 mol AgCl) = 104.6 g AgCl
Comparing the two amounts of product, we can see that the amount of AgCl that can be formed from the iron (III) chloride is greater than the amount that can be formed from the silver nitrate. Therefore, silver nitrate is the limiting reactant in this reaction.
Explanation:
Related Questions
A 35.40 gram hydrate of sodium carbonate, Na2CO3•nH2O, is heated to a constant mass. Its final weight is 30.2 g. What is formula for the hydrate?A. Na2CO3∙1H2OB. Na2CO3∙2H2OC. Na2CO3D. Na2CO3∙3H2O
Answers
First, we have to calculate the molecular weights of each molecule:
\(\begin{gathered} Na_2CO_3\text{ : 23*2+12+16*3= 106 g/mol} \\ H_2O\text{ : 1*2+16= 18 g/mol} \end{gathered}\)Then, we have to calculate the number of grams of water. We can calculate them because the process of evaporation lets us know the water amount that was retired:
\(g\text{ H}_2O\text{ = 35.40 g - 30.2 g=5.2 g H}_2O\)Then, we're gonna convert the grams of sodium carbonate alone (30.2 g) and the grams of water to moles:
\(\begin{gathered} 30.2\text{ g Na}_2CO_3\text{ * }\frac{1\text{ mol}}{106\text{ g}}=\text{ 0.2849 mol Na}_2CO_3\text{ }\approx0.3\text{ mol Na}_2CO_3 \\ \\ 5.2\text{ g H}_2O\text{ * }\frac{1\text{ mol}}{18\text{ g}}=\text{ 0.288 mol H}_2O\text{ }\approx\text{ 0.3 mol H}_2O \end{gathered}\)It means that the mole relation is 1:1 approx, as it is the same amount for both. Then, the formula is going to be:
\(Na_2CO_3\text{ . 1H}_2O\)It means that the answer is A.
A force caused by objects moving in opposite directions called
Answers
Answer: Balanced forces
Explanation: Equal forces acting in opposite directions are called balanced forces. Balanced forces acting on an object will not change the object's motion.
caso4 · 2h2o is a(n)answerbecause it always contains a fixed ratio of water molecules to calcium and sulfate ions.
Answers
The 2h2o stands for calcium sulfate dihydrate, which means it has two water molecules connected to the calcium sulfate crystal lattice.
The correct answer to the statement "caso4 · 2h2o is a hydrate because it always contains a fixed ratio of water molecules to calcium and sulfate ions" is hydrate.
What is a hydrate?
A hydrate is a crystalline compound that includes water molecules in its composition. The water molecules are included as part of the crystal lattice, which means they are connected to the ions in the compound through hydrogen bonding.
The water molecules are usually eliminated from the hydrate when it is heated, resulting in an anhydrous compound.\A hydrate is characterized by a specific ratio of water molecules to the number of ions in the compound, and this ratio is constant throughout the substance.
Therefore, caso4 · 2h2o is a hydrate because it always contains a fixed ratio of water molecules to calcium and sulfate ions, as stated in the question.
In this case, caso4 ·
2h2o stands for calcium sulfate dihydrate, which means it has two water molecules connected to the calcium sulfate crystal lattice.
Learn more about calcium with the given link,
https://brainly.com/question/26636816
#SPJ11
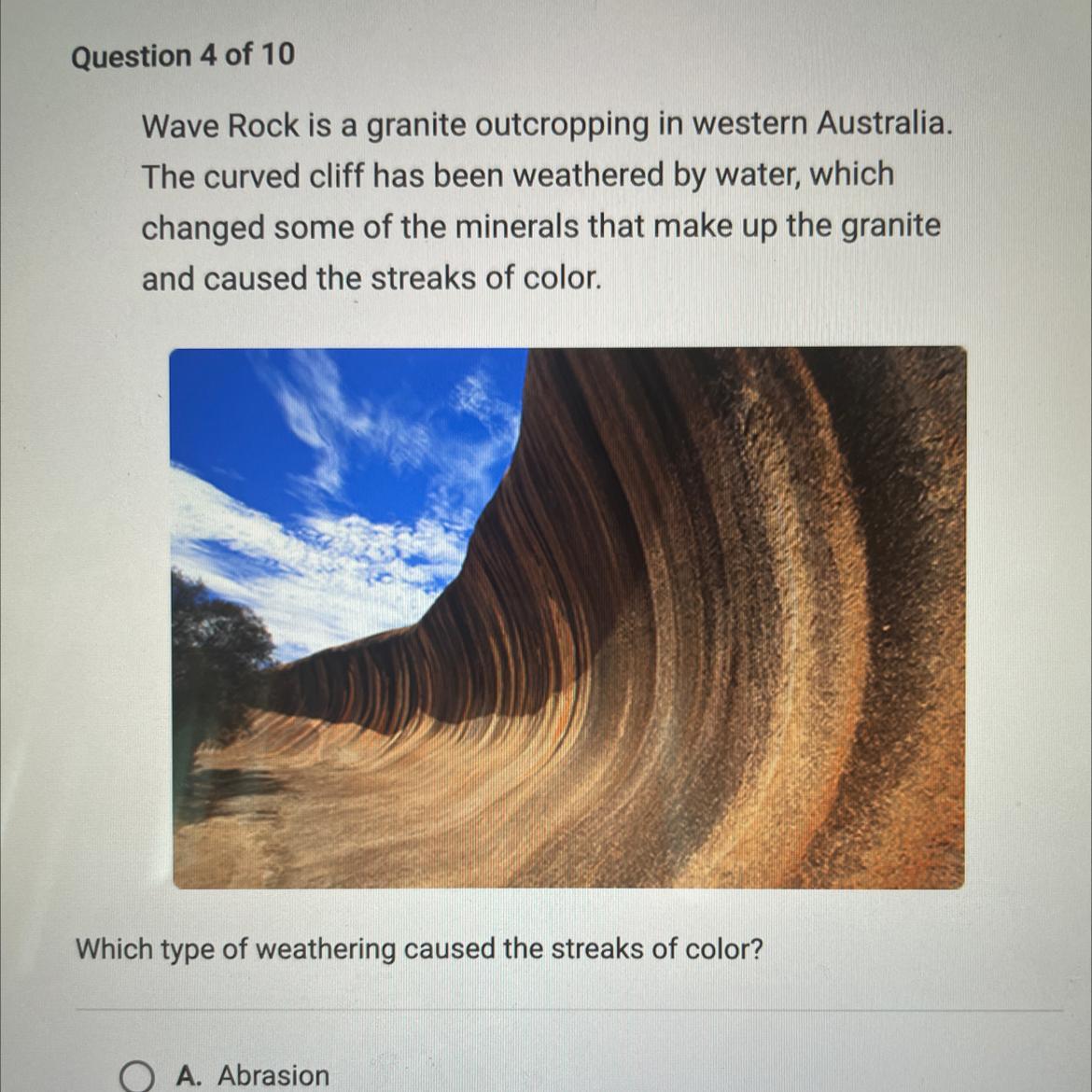
Wave Rock is a granite outcropping in western Australia.
The curved cliff has been weathered by water, which
changed some of the minerals that make up the granite
and caused the streaks of color.
Which type of weathering caused the streaks of color?
Answers
The type of weathering caused by the streaks of color is chemical weathering.
What is chemical weathering of the rock about?Chemical weathering occurs when the minerals in a rock are chemically altered by exposure to water, acids, or other chemicals. This process can change the composition of the rock and cause it to take on new colors or patterns.
In the case of Wave Rock, it is likely that the streaks of color were caused by the alteration of minerals in the granite by water. Water can dissolve minerals in rocks and cause them to leach out, leaving behind new minerals that may have different colors. This process can result in the formation of streaks or patterns on the surface of the rock.
Note that Physical weathering, on the other hand, occurs when the physical structure of a rock is changed by mechanical forces such as freezing and thawing, abrasion, or pressure. This type of weathering does not typically result in changes to the chemical composition of the rock, and is not likely to be the cause of the streaks of color on Wave Rock.
Learn more about chemical weathering from
https://brainly.com/question/29784758
#SPJ1
what happened to the shape of the water when it was poured into a bottles water
Answers
Answer:
Its still a liquid, only it takes form of the bottle.
Explanation:
When you pour a liquid (water) in a bottle, the water is still water but the water takes shape of the bottle. Hope this helps! Let me know if you need clarification.
If a solution has a [H+] of 9.3x10-11, what is the pH?
Answers
Answer:
10.03Explanation:
The pH of a solution can be found by using the formula
\(pH = - log [ {H}^{+} ]\)
From the question we have
\(ph = - log(9.3 \times {10}^{ - 11} ) \\ = 10.0315...\)
We have the final answer as
10.03Hope this helps you
Fill in the blanks: a substance that undergoes a ______ change will always result in something new that has ________ properties than what it started out as. A- physical and different. b- physical and same. C-chemical and different. D chemical and same
Answers
(s)-2-methylbutanal ________ upon sitting in an acidic or a basic aqueous solution. a. inverts completely to the r configuration
Answers
(s)-2-methylbutanal undergoes racemization in both acidic and basic aqueous solutions, resulting in the formation of a racemic mixture containing both (s)- and (r)-2-methylbutanal.
(s)-2-methylbutanal undergoes racemization upon sitting in an acidic or a basic aqueous solution. Racemization refers to the conversion of a pure enantiomer into a racemic mixture, which contains equal amounts of both enantiomers. In this case, the (s)-2-methylbutanal would convert into a mixture of both (s)- and (r)-2-methylbutanal.
The racemization process occurs due to the reversible interconversion of enantiomers. In an aqueous solution, the presence of acid or base can facilitate this interconversion by breaking and forming chemical bonds. The acid or base catalyzes the racemization reaction, allowing the (s)-2-methylbutanal to convert into its enantiomer, (r)-2-methylbutanal.
It is important to note that racemization is a dynamic process, meaning that the enantiomers are continuously interconverting as long as the reaction conditions are maintained. The rate of racemization can be influenced by factors such as temperature, concentration, and the presence of catalysts.
Learn more about racemization here:-
https://brainly.com/question/31665891
#SPJ11
calculate the ph of a solution having [OH-] = 8.2 x 10 ^-8M
Answers
Answer:
6.91Explanation:
To find the pH we must first find the pOH
The pOH can be found by using the formula
pOH = - log [ {OH}^{-} ]
We have
\(pOH = - log(8.2 \times {10}^{ - 8} ) \\ = 7.086186...\)
pOH = 7.09
Next we use the equation
pH = 14 - pOH
We have
pH = 14 - 7.09
We have the final answer as
6.91Hope this helps you
Which of these has an oxidation number of zero (0)? Cal2, Ca CaS, Ca3N2
Answers
Ca is the correct answer. Maybe
calculate the volume of carbon (iv) oxide measured at S.T.P that is evolved when 1 mole of copper (ii) carbonate is heated to consant mass?
Answers
The volume of carbon (iv) oxide measured at S.T.P. that is evolved when one mole of copper (ii) carbonate is heated to constant mass is 22.4 L.
What is the volume of carbon dioxide produced?The volume of carbon dioxide produced when one mole of copper (ii) carbonate is heated to constant mass is given by the equation of the reaction given below;
CuCO₃ (s) ----> CuO (s) + CO₂ (g)
The mole ratio of the reaction shown above is 1 : 1 for copper carbonate and carbon dioxide.
From the equation of the reaction, one mole of copper carbonate when heated to a constant mass produces one mole of carbon dioxide.
The volume of one mole of carbon dioxide is equal to the volume of one mole of a gas.
The volume of one mole of a gas at STP is 22.4 L
Hence the volume of carbon dioxide evolved is 22.4 L
Learn more about the molar volume of a gas at: https://brainly.com/question/1496714
#SPJ1
what does Le châteliers principle state?
Answers
Hope this helps!
A bar which contains a uniform concentration of 5 atomic percent Cr has its surface coated with pure Cr. When the bar is exposed to high temperature, at a point within the original bar but near the surface, the concentration of the Cr will generally ______________ with time.
Answers
A bar which contains a uniform concentration of 5 atomic percent Cr has its surface coated with pure Cr. When the bar is exposed to high temperature, at a point within the original bar but near the surface, the concentration of the Cr will generally Increase with time.
When the bar is exposed to high temperature, the pure Cr coating on the surface will diffuse into the bar, leading to an increase in the concentration of Cr within the bar near the surface. This diffusion process is driven by the concentration gradient between the surface and the interior of the bar. Over time, the concentration of Cr within the bar will become more uniform, but it will still be higher near the surface due to the diffusion of the pure Cr coating. Therefore, the concentration of Cr within the bar will generally increase with time.
learn more about Cr coating
https://brainly.com/question/31604696
#SPJ11
how to tell if a functional group is acidic or basic
Answers
Determining whether a functional group is acidic or basic depends on its ability to either donate or accept a proton (H+). Here are some general guidelines to help you assess the acidity or basicity of a functional group:
1. Acidity:
a. Look for functional groups that have an acidic hydrogen directly bonded to an electronegative atom, such as oxygen or a halogen. Examples include carboxylic acids (–COOH) and phenols (–OH on an aromatic ring).
b. Consider the stability of the resulting conjugate base. If the conjugate base is stabilized through resonance or delocalization of the negative charge, the functional group is more acidic. For example, the carboxylate ion (–COO-) is stabilized through resonance.
2. Basicity:
a. Look for functional groups that contain lone pairs of electrons, which can readily accept a proton. Common examples include amines (–NH2) and amides (–CONH2).
b. Consider the availability of lone pairs. The more accessible the lone pairs are, the more basic the functional group. For example, primary amines have more available lone pairs than tertiary amines and are, therefore, more basic.
It's important to note that the acidity or basicity of a functional group can also be influenced by its environment, neighboring groups, and other factors. These guidelines provide a general starting point, but there may be exceptions and variations based on specific compounds and circumstances.
To know more about functional group refer here
https://brainly.com/question/31332495#
#SPJ11
Scenario 1: Sarah, your lab partner, accidentally poured too much
sodium chloride (chemical) into a beaker. She wants to pour the rest back
into the original container. What should you do? Explain
Scenario 2: You notice that yout lab partner is chewing gum and using a
beaker as a cup to get water. He then takes a drink from the beaker. He
assures you that it is clean and you don't have to worry. Why is this
dangerous in lab? Explain.
Scenario 3: In lab, you are working with an open flame (a birthday
candle) and your friend calls you over to see new background picture on
his chromebook. What should you do? Explain
Answers
Scenario 2: chemicals have been on beakers and they should not be drank out of, your partner should also not be eating or drinking in the lab.
Scenario 3: you should never walk away from an open flame
the overall take away of an experiment’s results is the______. A. hypothesis B. introduction C. conclusion
Answers
Answer:
conclusion
Explanation:
it can't be a hypothesis since tests are carried out to verify so it is not a theory
an introduction to an experiment only gives the basis of what we are investigating therefore nothing has been proven and the question is still unanswered
The overall takeaway of an experiment’s results is the conclusion. Hence, option C is correct.
What is a hypothesis?A hypothesis is a testable statement about the relationship between two or more variables or a proposed explanation for some observed experiment.
The overall takeaway of an experiment’s results can't be a hypothesis since tests are carried out to verify so it is not a theory
An experiment only gives an idea about the investigation therefore nothing has been proven and the question is still unanswered.
Hence, the overall takeaway of an experiment’s results is the conclusion.
Learn more about the hypothesis here:
brainly.com/question/5177511
#SPJ5
Kinetic energy CANNOT be converted into potential energy
True
False
Answers
Answer:
false
Explanation:
Answer:
False
Explanation:
Because if you shoot a arrow and go back and get it and use it again it is potential
which of the following statement(s) is/are true about chemical kinetics? group of answer choices chemical kinetics shows the transfer of energy in the form of heat and/or work. chemical kinetics is the branch of physical chemistry that is concerned with the direction in which a process occurs but in itself tells nothing about its rate. a reaction rate law must match the coefficients in the balanced chemical equation reaction rate can be defined as the decrease in the concentrations of reactants with time. chemical kinetics is the study of the rates of reactions.
Answers
The following two statements are true about chemical kinetics.
1. Chemical kinetics is the study of the rate of reactions.
2. A reaction rate can be defined as the decrease in the concentrations of reactants with time.
Chemical kinetics deals with the study of rate of chemical reactions. Rate of reaction is defined as the measure of the change in concentration of the reactants or the change in concentration of the products per unit time.
The reaction rates decrease with time because reactant concentrations decrease as reactants are converted to products. The rate decreases as the reaction proceeds.
A→B
A= reactant rate of reaction =-\(\frac{d[A]}{dt}\)
B=product rate of reaction=+\(\frac{d[B]}{dt}\)
This means that the concentration of 'A' decrease with respect to time. It can also be noted by measuring the increase in the Concentration of B with the passage of time.
Negative sign indicates the conc. of reactants decreases with time
Positive sign indicates that conc. of product increases with the passage of time .Remember rate of reaction always positive.
To learn more about Chemical Kinetics, click here
brainly.com/question/28940590
#SPJ4
The reactant side of a balanced chemical equation is shown below.
Al2(SO4)3 + 3Cl2 →
How many chlorine atoms should there be on the product side in the equation?
2
5
6
7
Answers
Answer:
2
Explanation:
In the equation, Cl is chlorine and there is a number "2" so that the correct answer is 2.
Answer:
two
Explanation:
The water has a mass of 480.0 grams. If you drink 200.0 grams of the water, how many molecules of water remain in the glass?
Answers
Answer:
55641.656 × 10^23 molecules
Explanation:
Get the molar mass, multiply by amount of stuff, multiply by Avogadros number.
More detail in picture attached.
Hope it helps. Brainliest Appreciated. :)

Why do your feet stay on the ground instead of floating?
Answers
Answer: because of gravity
Explanation: gravity is the thing that holds us on the earth. without it, we would be dead and our bodies would be floating in space.
Answer:Gravity
Explanation: It pull you back down
Reduction is the gain of electrons and oxidation is the loss of electrons. Is the hydrogen molecule being oxidized or reduced in the fuel cell?
Answers
The hydrogen molecule is being oxidized in the fuel cell.
What is happening to the hydrogen molecule in the fuel cell? Is it being oxidized or reduced?In a fuel cell, the hydrogen molecule (H₂) acts as the fuel. During the operation of a fuel cell, a redox (reduction-oxidation) reaction takes place. In this reaction, hydrogen is oxidized, which means it loses electrons. The oxidation of hydrogen occurs at the anode of the fuel cell.
In a fuel cell, hydrogen gas is supplied to the anode, where it is split into protons (H⁺) and electrons (e⁻) through a process called electrolysis. The electrons are then forced to travel through an external circuit, creating an electric current that can be used to power devices. Meanwhile, the protons move through a proton exchange membrane (PEM) to the cathode.
At the cathode, an oxidizing agent (typically oxygen from the air) combines with the electrons and protons to form water (H₂O). This reduction process at the cathode involves the gain of electrons by the oxidizing agent, which in this case is oxygen. Therefore, the reduction of oxygen is occurring at the cathode.
The hydrogen molecule is being oxidized in the fuel cell, meaning it is losing electrons. This oxidation process provides the electrons necessary for the reduction of the oxidizing agent, which is typically oxygen.
Learn more about Fuel cell
brainly.com/question/15168404
#SPJ11
TOMMY (persistently but a little intimidated by the crowd) It's always that way, in every story I ever read about a ship landing from outer space. —The Monsters Are Due on Maple Street, Rod Serling What scientific fact is Tommy’s theory based on? Meteors can do some crazy things. Spaceships often land on Earth. Technology makes space travel possible. Meteors can move people from planet to planet.
Answers
Answer:
Technology makes space travel possible.
Explanation:
bc the guy above me is wrong I had to guess and I got it right
Answer:
Technology makes space travel possible.
Explanation:
got it right on edge
5. What is the primary purpose of muscle?
Answers
Answer:
Any type of movement
Explanation:
All muscles are used for any way of movement in the body.
the most stable compound shown is
A. N 2
B. H 2O(g)
C. NH 3(g)
D. C (g)

Answers
H₂O(g) is the most stable compound of all the given compounds.
What is Enthalpy?The concept of enthalpy pertains to the heat content of a given system under constant pressure, and is denoted by the letter "H". It is a fundamental thermodynamic property that can be expressed as the sum of a system's internal energy and the product obtained by multiplying its pressure with volume. In simpler terms, enthalpy provides insight into how much heat energy is present within a system at any given point in time.
Which is the most stable compound?Out of the options provided, H₂O(g) or water vapor is the most stable compound in terms of enthalpy rate. The reason being, water has a negative enthalpy of formation which implies that energy is released when it is formed from its constituent elements - hydrogen and oxygen. This negative enthalpy value signifies that water is thermodynamically stable at standard conditions.
While N₂ also has a negative enthalpy of formation, it is comparatively less negative than that of water, which means it is less stable than water. On the other hand, both NH₃ and C(g) have positive enthalpies of formation indicating they are less stable than their constituent elements and require energy for their formation. Therefore, based on these factors, H₂O(g) emerges as the most stable compound among all the given options.
To know more about enthalpy, click here
https://brainly.com/question/13996238
#SPJ1
below is one of the lewis structures of so3, which has two other resonance structures that do not violate the octet rule. complete one of the other resonance structures by dragging bonds and electron lone pairs to their appropriate positions. then click check.
Answers
For SO3, there are seven resonance structures. For sulfur dioxide, also known as SO2, there are two resonance structures that both contribute equally to the molecule's overall hybrid structure.
How many SO3 pairs lack bonds?According to the Lewis structure, there are therefore zero lone pairs and three sigma bonds in a single SO3 molecule.
Is SO3 covered by the octet rule?Dative bonds can be found in SO3 according to the octet rule.
Which of the Lewis structures cannot be drawn in accordance with the octet rule?Around the Se atom, there are eight electrons that bond together and one electron pair that doesn't. The structure of Lewis is: As a result, it is impossible to derive a valid Lewis structure for SeF4 S e F 4 without breaking the octet rules.
To learn more about hybrid structure here:
https://brainly.com/question/13829121
#SPJ4
a) Water has a molar mass of 18 g/mol and a density of 1000 kg/m3 (or 1 g/cm3). Based on this data, estimate the number of water molecules per unit surface area of water.
b) The coordination number of water (i.e., the average number of "neighbors" each water molecule has) in the liquid state is 4. Neighboring water molecules attract each other via hydrogen bonds, each of which has a binding energy of roughly 10–20 J (although this number depends relatively strongly on temperature). Use this information to estimate the surface tension of water. How does your estimate compare to the observed figure (
γ water = 0.072 N/m ) (hints: Keep in mind that we can think of
surface tension as surface energy per unit area, and consider the energy needed to bring a molecule from the bulk to the surface)?
Answers
The estimate is still useful because it provides insight into the behavior of water molecules at the surface of the liquid.
Molar mass of water, M = 18g/molDensity of water, ρ = 1g/cm³ = 1000kg/m³The number of molecules per unit surface area of water can be estimated as follows:Number of water molecules per unit volume of water = Avogadro's number, NA / MNumber of water molecules per unit volume of water = 6.022 × 10²³ / 18 = 3.345 × 10²² / molThe number of molecules per unit surface area of water = the number of molecules per unit volume of water × the thickness of the water layer on the surface= 3.345 × 10²² / m³ × 1 × 10⁻⁸ m= 3.345 × 10¹⁴ / m²b)Given:Coordination number of water, CN = 4Binding energy of hydrogen bond, E = 10⁻²⁰ JThe surface tension of water, γ water = 0.072 N/mEnergy required to bring one molecule from the bulk of the liquid to the surface of the liquid, ΔE= γ water × AThe total binding energy of a water molecule in the liquid state = the binding energy of one hydrogen bond × the coordination number= 10⁻²⁰ J/bond × 4 bonds = 4 × 10⁻²⁰ JThe number of molecules per unit surface area of water = the energy required to bring one molecule from the bulk of the liquid to the surface of the liquid / the total binding energy of a water molecule in the liquid state= ΔE / 4 × 10⁻²⁰= 0.072 / (4 × 10⁻²⁰)= 1.8 × 10²⁰The surface tension of water can also be expressed as follows:γ water = (N / A) × EThe number of hydrogen bonds per unit area, N / A = γ water / E= 0.072 / 10⁻²⁰ = 7.2 × 10¹⁸ / m²The difference between the estimated value and the observed value is relatively large (about a factor of 25). It is because this is just an estimate, and it does not consider all the factors affecting the surface tension of water. However, the estimate is still useful because it provides insight into the behavior of water molecules at the surface of the liquid.
Learn more about liquid here:
https://brainly.com/question/20922015
#SPJ11
Without any calculations, determine which solution in each pair is more basic.
Part A
a.0.100 M in KClO
b. 0.100 M in NaF
Part B
a. 0.0100 M in NaBrO
b. 0.0100 M in NaBr
Part C
a. 0.0100 M in HNO_2
b. 0.0100 M in KOH
Part D
a. 0.0100 M in NH_4Cl
b. 0.0100 M in HCN
Answers
In each pair, the solution that contains the weaker conjugate acid is more basic. Without any calculations, we can determine which solution is more basic by identifying the stronger conjugate acid in each pair.
In Part A, KClO is a stronger acid than NaF, so the solution in (b) is more basic in Part B, NaBrO is a stronger acid than NaBr, so the solution in (b) is more basic in Part C, HNO2 is a weaker acid than KOH, so the solution in (b) is more basic in Part D, NH4Cl is a weaker acid than HCN, so the solution in (a) is more basic.
It is important to note that while we did not perform any calculations, this method only works for comparing solutions with the same concentration. If the concentrations were different, we would need to perform calculations to determine which solution is more basic. A
To know more about conjugate acid visit:
https://brainly.com/question/31229565
#SPJ11
Rewrite the molality expression in terms of grams and molecular weight. Please show the derivation from the general expression in all the steps.
Answers
I'm happy to help you rewrite the molality expression in terms of grams and molecular weight. Here's the derivation from the general expression, step by step:
Step 1: Define molality
Molality (m) is the number of moles of solute per kilogram of solvent. The general expression for molality is:
m = (moles of solute) / (mass of solvent in kg)
Step 2: Convert moles to grams
To rewrite the expression in terms of grams, we need to replace the moles of solute with grams. We can do this using the molecular weight (MW) of the solute:
moles of solute = (grams of solute) / (molecular weight of solute)
Step 3: Substitute the conversion
Now, substitute this conversion into the general molality expression:
m = [(grams of solute) / (molecular weight of solute)] / (mass of solvent in kg)
Step 4: Rewrite the expression
Finally, we can rewrite the expression as:
m = (grams of solute) / [(molecular weight of solute) * (mass of solvent in kg)]
And that's the molality expression in terms of grams and molecular weight.
To know more about molality refer here:
https://brainly.com/question/26921570#
#SPJ11
For the reaction ?FeCl2 + ?Na3PO4 → ?Fe3(PO4)2 + ?NaCl ,
what is the maximum number of moles of Fe3(PO4)2 which could be formed from
7.23 mol of FeCl2 and 4.39 mol of Na3PO4? Answer in units of mol.
Answers
The maximum number of moles of Fe3(PO4)2 that can be formed is 0.807 mol when 7.23 mol of FeCl2 and 4.39 mol of Na3PO4 are present.
In the given reaction, we have to find the maximum number of moles of Fe3(PO4)2 that can be formed using 7.23 mol of FeCl2 and 4.39 mol of Na3PO4.Reaction: FeCl2 + Na3PO4 → Fe3(PO4)2 + NaClWe will balance the given chemical equation to get the balanced chemical equation. FeCl2 + 3Na3PO4 → Fe3(PO4)2 + 6NaClThe balanced chemical equation is given above. Now we will use stoichiometry to solve the question.The molar ratio of FeCl2 to Fe3(PO4)2 is 1:1 from the balanced chemical equation.The molar ratio of Na3PO4 to Fe3(PO4)2 is 3:1 from the balanced chemical equation.Using the molar ratios and the given number of moles, we can calculate the maximum number of moles of Fe3(PO4)2 that can be formed.Let x be the number of moles of Fe3(PO4)2 formed.
According to the balanced chemical equation, moles of FeCl2 react with moles of Na3PO4 to form moles of Fe3(PO4)2.So, from the given number of moles of FeCl2, the number of moles of Fe3(PO4)2 formed is:x = 7.23 mol of FeCl2 × (1 mol Fe3(PO4)2/1 mol FeCl2)×(1 mol Na3PO4/3 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 0.807 mol of Fe3(PO4)2Using the given number of moles of Na3PO4, the number of moles of Fe3(PO4)2 formed is:x = 4.39 mol of Na3PO4 × (1 mol Fe3(PO4)2/3 mol Na3PO4)×(1 mol FeCl2/1 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 1.463 mol of Fe3(PO4)2.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8