Stomic size decreases across a period due to an increase in the effective nuclear charge, zeff. group of answer choices true false
Answers
The given statement "atomic size decreases across a period due to an increase in the effective nuclear charge, Zeff" is true because Zeff causes the valence electrons to be more strongly attracted to the nucleus resulting in decreased atomic size.
As you move across a period, the number of valence electrons in the outermost shell increases, but the number of energy levels or shells remains the same. This means that the electrons are being added to the same shell and are therefore at roughly the same distance from the nucleus. However, the effective nuclear charge (Zeff) increases across the period due to the increasing number of protons in the nucleus. This causes the valence electrons to be more strongly attracted to the nucleus and held more tightly, resulting in a decrease in atomic size across the period.
Therefore the given statement "atomic size decreases across a period due to an increase in the effective nuclear charge, Zeff" is true.
To learn more about periodic properties refer to: https://brainly.com/question/31232139
#SPJ11
Related Questions
A bromine substituent on the benzene ring _______________it and makes it _________ reactive than benzene molecule.
Answers
A bromine substituent on the benzene ring decreases its reactivity compared to the benzene molecule.
When a bromine atom is substituted onto the benzene ring, it exerts an electronic effect that influences the reactivity of the compound. Bromine is an electron-withdrawing group, meaning it attracts electrons towards itself. This occurs because bromine is more electronegative than carbon.
As a result, the presence of the bromine atom withdraws electron density from the benzene ring, creating a partial positive charge on the carbon atoms adjacent to the bromine. This electronic effect decreases the reactivity of the benzene ring.
Due to the electron-withdrawing nature of the bromine substituent, the benzene ring becomes less reactive towards electrophilic aromatic substitution reactions. Electrophilic aromatic substitution reactions involve the attack of electrophiles (electron-deficient species) on the benzene ring, where the electrophile replaces a hydrogen atom.
In the presence of a bromine substituent, the electron density on the ring is reduced, making it less attractive to electrophiles. Consequently, the reaction rate is slower, and the overall reactivity is diminished.
Learn more about Benzene ring
brainly.com/question/31490176
#SPJ11
We have a cylinder. Calculate the following:
What is the cylinder's mass?
Data:
Volume: 376.8m3
Mass: x
Density: 1000km/m3
Answers
Answer:
mass = 2.65 [nearest hundredth].
Explanation:
Mass = Density / Volume
mass = 1000 / 376.8
mass = 2.653927813163.. ≈ 2.65
Are metals fcc or BCC?
Answers
A Face Center Cubic Structure (fig. la), a Body Centered Cubic Structure (fig. lb), or an Hexagonal Close Packed structure (fig. lc) characterizes the majority of common metals.
Metal crystal structures, also known as the cubic-closest-packed (CCP) or face-centered cubic (FCC) lattice. In fcc lattices, copper, silver (Ag), and gold (Au) crystallize. The closest feasible packing of spheres can be seen in the hcp and fcc structures, where spheres occupy 74% of the volume.
One atom in the middle and eight eighths from each corner make up the BCC unit cell's total of two atoms. The eight atoms at the unit cell's corners and the single atom positioned in the center of each face are present in the FCC layout as well. With the neighboring cell, the atom in the face is shared.
Despite having more slip systems than FCC, the slip planes of BCC materials are not as densely packed. As a result, FCC materials are more ductile than BCC and have a tendency to distort more easily. The BCC materials are more durable and robust than the FCC ones.
To know more about metals fcc or BCC, click on the link below:
https://brainly.com/question/21282258
#SPJ4
how many electrons each atom gives or takes in KBr
Answers
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
What is the volume of the water?
0 points
Captionless Image

Answers
Answer:
the value of water is
6000
Answer:
600 or 6000 I'm not sure
the electrolyte in automobile lead storage batteries is a 3.75 sulfuric acid solution that has density of 1.230g/mL. calculate the mole fraction.
Answers
The mole fraction of sulfuric acid : 0.073
Further explanationGiven
3.75 M sulfuric acid solution that has density of 1.230 g/mL
Required
The mole fraction
Solution
3.75 M = 3.75 mol/L
For 1 L solution :
mass of solution = 1230 g
mass Sulfuric acid : 3.75 mol x 98 g/mol=367.5 g
mass water = 1230-367.5 = 862.5 g
mol water = 862.5 : 18 g/mol= 47.92
mol total = 3.75 + 47.92 = 51.67
mole fraction of sulfuric acid :
= 3.75 : 51.67
= 0.073
what type of fire is self-heating? diffusion flame spontaneous combustion pre-mixed flame smoldering
Answers
The type of fire that is self-heating is spontaneous combustion. This occurs when a combustible material (such as paper, rags, oil, and even coal) is heated to its ignition temperature through a process of oxidation. The heat produced from the oxidation will then cause the material to burn.
combustion refers to a fire that starts on its own due to heat generated by chemical reactions rather than an external source. This type of fire typically happens in organic materials that produce heat during natural decay, such as hay or wood chips that have been compressed or stored in large quantities. When the heat produced by these chemical reactions surpasses the material's ability to dissipate it, the temperature will keep rising until the material catches fire. The other types of fire are as follows:
Diffusion flame A diffusion flame is a fire that occurs when a fuel source is mixed with air and burned in the presence of an oxidizer. Diffusion flames are common in industry and can be found in applications like boilers, furnaces, and power plants
.Premixed flame A pre-mixed flame is a type of flame that occurs when a fuel source is mixed with air before it is ignited. This type of flame is often used in internal combustion engines.
Smoldering A smoldering fire is a slow, low-temperature flame that occurs when materials like embers or coals continue to burn without visible flame. This type of fire is often found in wildfires, and it can be very dangerous because it can spread underground or in places where people might not see it.
For more such questions on spontaneous combustion , Visit:
https://brainly.com/question/30392290
#SPJ11
A balloon is inflated to 665 mL volume at 27°C. It is then cooled down to -78.5°C. What
is its volume, assuming the pressure remains constant?
Answers
Answer:
431 mL
Explanation:
This is a question about the relationship between the volume and temperature of a gas. The volume of a gas is directly proportional to its temperature in kelvins when the pressure is held constant. This relationship is described by Charles’s Law.
To solve this problem, we need to convert the temperatures from degrees Celsius to kelvins by adding 273.15. So 27°C is equivalent to 300.15 K and -78.5°C is equivalent to 194.65 K.
Let’s call the initial volume of the balloon V1 and its initial temperature T1. The final volume of the balloon will be V2 and its final temperature T2. According to Charles’s Law, the relationship between these variables can be expressed as:
V1/T1 = V2/T2
Substituting the known values into this equation, we get:
665 mL / 300.15 K = V2 / 194.65 K
Solving for V2, we find that the final volume of the balloon is approximately 431 mL.
Chromogen can have----------- Positive Charg,e Negative Charge ,Both A and B ,No Charge/Neutral
Answers
Chromogen can have positive and negative charge which is option C.
Chromogen explained
A chromogen is something that can change color when it goes through a reaction or touch something else. In science, chromogens are used to help analyze things like colors or stains in chemicals and living things.
Chromogens are things that can be organic or not and they make colors. Why they make colors can be because of different things, like when atoms move or when they combine in ways that make it happen. The color we see from a chromogen happens because of how it reflects or takes in light waves, based on its chemical structure or when it combines with other things.
Learn more about chromogen below.
https://brainly.com/question/30053184
#SPJ4
Fill in the table below with your results from Part C. Test reagent Equilibrium Direction Primary NIE AgNO NaNO3 NH4OH (NH4)2C204 Na3PO4 Would you expect any of the test reagents from Part C to change the equilibrium constant? Explain
Answers
Hi! I can provide an explanation on the topic without specific results from Part C, as I don't have access to that data. In a chemical equilibrium, the equilibrium constant (K) is a measure of how far the reaction proceeds before reaching equilibrium. When you add test reagents, they can shift the equilibrium in either direction, but they do not change the equilibrium constant (K) itself. The equilibrium constant remains constant for a given reaction at a specific temperature. From the test reagents mentioned: AgNO₃, NaNO₃, NH₄OH, (NH₄)₂C₂O₄, and Na₃PO₄, any potential shifts in equilibrium direction would depend on the chemical reaction involved. However, these shifts would not alter the equilibrium constant (K) as it is solely dependent on temperature. To summarize, the test reagents from Part C may shift the equilibrium direction, but they will not change the equilibrium constant.
About EquilibriumEquilibrium It is a state of balance between opposing forces or actions that is either static (as in a body acted on by forces whose resultant is zero) or dynamic (as in a reversible chemical reaction when the rates of reaction in both directions are equal). Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degree. For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
Learn more about equilibrium at https://brainly.com/question/517289
#SPJ11
a carbonate ion, co32-, can participate in an acid-base reaction. how should the carbonate ion be classified?
Answers
The carbonate ion is classified as amphiprotic, meaning it can act as both an acid and a base in a chemical reaction.
The carbonate ion (CO₃²⁻) is a polyatomic ion composed of one carbon atom and three oxygen atoms. It has a negative charge of 2⁻, which is balanced by one or more positive ions in ionic compounds.
The carbonate ion is a versatile species that participates in various chemical reactions. For example, it can react with acids to form bicarbonate (HCO₃⁻) or carbonic acid (H₂CO₃). It is also a key component of many minerals, such as limestone, calcite, and dolomite.
The carbonate ion, CO₃²⁻, can participate in an acid-base reaction and can act as a base, accepting a proton from an acid to form bicarbonate (HCO₃⁻) or as an acid, donating a proton to a base to form carbonic acid (H₂CO₃).
To know more about carbonate ion here
https://brainly.com/question/13878773
#SPJ4
1. Which location (a, b, or c) would have the highest temperature?
2. Which location(a, b, or c) would have the lowest temperature?
3. Give reasoning to explain how and why the latitude of a place on Earth affects its average temperature.
WORTH 100 POINTS! WILL MARK BRIANLESIT
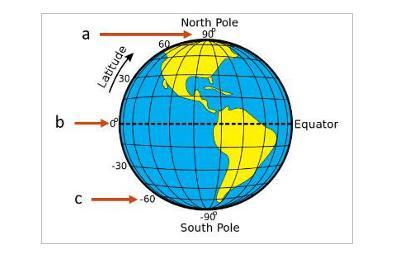
Answers
Answer:
1. B
2.C
3.The further out and the closer the the equator a place on Earth is the warmer it is due to the fact that the sun directly hits countries on the equator and takes in heat faster then the north and south pole. Countries on more of a curve are colder due to the fact they are more curved and it is harder for the heat to be taken in at a curve.
B is center point line passing through earth and it's placed right infront of sun so b would have highest temperature
#2
A and C both are poles so at a and c least sun light can reach hence lowest temperature.
#3
As far as the latitude is placed from equator lower the temperatureAs near as the latitude is located from equator higher the temperatureI need help please!!

Answers
Answer:
BECAUSE OF DENSITY
Explanation:
Answer:
It's DENSITY
Explanation:
YOURE WELCOME
What volume would be needed to prepare 375 mL of a .45 M CaCl2 using only a solution of 1.0 M CaCl2 and water?
Answers
Answer:
168.75 ml
Explanation:
M1V1=M2V2
375ml*.45M=1M*V2
Briefly explain why is it important for science teachers to know how to prepare stock solution
Answers
It is important for science teachers to know how to prepare stock solutions because stock solutions are concentrated solutions that can be used to make various dilutions for experiments and scientific investigations.
1. Consistency and accuracy: Stock solutions ensure consistency and accuracy in scientific experiments. They provide a known concentration of a specific substance, allowing teachers to prepare solutions with precise and reproducible concentrations. This is crucial for obtaining reliable and consistent results.
2. Time and resource efficiency: Stock solutions save time and resources by eliminating the need to measure and prepare individual solutions for each experiment. Once a stock solution is prepared, it can be used multiple times for different experiments or dilutions. This makes the teaching process more efficient and allows for more experiments to be conducted within a limited time frame.
3. Safety: Stock solutions are often highly concentrated and may contain hazardous substances. Teachers need to know how to properly handle, store, and dilute stock solutions to ensure the safety of themselves and their students.
By understanding the proper procedures for preparing and handling stock solutions, teachers can create an optimal learning environment for their students and facilitate meaningful scientific investigations.
learn more about stock solutions
https://brainly.com/question/3942978
#SPJ11
balance the equation. what mass, in grams, of carbon dioxide is formed by the complete combustion of 39.0 g of benzene?
Answers
132.03 g of carbon dioxide is formed by the complete combustion of 39.0 g of benzene.
The balanced equation for the complete combustion of benzene is:
C6H6 + 15/2 O2 → 6 CO2 + 3 H2O
From the equation, we can see that 1 mole of benzene (C6H6) reacts with 15/2 moles of O2 to produce 6 moles of CO2.
First, we need to find the number of moles of benzene in 39.0 g:
n = m/M
n = 39.0 g / 78.11 g/mol (the molar mass of benzene)
n = 0.500 mol
Next, we use the mole ratio between benzene and CO2 to find the number of moles of CO2 produced:
n(CO2) = n(C6H6) x (6 mol CO2 / 1 mol C6H6)
n(CO2) = 0.500 mol x (6 mol CO2 / 1 mol C6H6)
n(CO2) = 3.00 mol CO2
Finally, we use the molar mass of carbon dioxide to convert the number of moles to grams:
m(CO2) = n(CO2) x M(CO2)
m(CO2) = 3.00 mol x 44.01 g/mol
m(CO2) = 132.03 g
For more question on benzene click on
https://brainly.com/question/4595986
#SPJ11
The accepted value for the boiling pont of water is 100°C. During an experiment, students recorded the temperature observations losted below. Which one is the most accurate temperature for the boiling point of water?
A- 95°C
B- 102°C
C- 103°C
D- 97°C
Answers
Answer:B
Explanation:
The accepted value for the boiling point of water is 100°C. During an experiment, students recorded the temperature observations listed below. The most accurate temperature for the boiling point of water would be 102°C, therefore the correct answer is option B.
What is the boiling point?It is a temperature at which the pressure exerted by the external atmosphere is equal to the vapor pressure of the substance.
As given in the problem The accepted value for the boiling point of water is 100°C. During an experiment, students recorded the temperature observations listed below. The most accurate temperature for the boiling point of water would be 102°C.
The standard number for water's boiling point is 100 °C. Students observed the temperatures stated below during an experiment since 102°C is the temperature that would most accurately represent the boiling point of water.
Thus, the correct response is option B since 102°C is the temperature that would most accurately represent the boiling point of water.
Learn more about the boiling point from here,
brainly.com/question/25777663
#SPJ2
What is the structural formula of 4-methyl pentan-2-ol
Answers
The 4-methyl pentane-2-ol (\(C_6H_{14}O\)) is an alcohol compound with a methyl group attached to the fourth carbon atom and a hydroxyl group attached to the second carbon atom in a five-carbon chain.
The structural formula of 4-methyl pentane-2-ol is \(C_6H_{14}O\). This is an alcohol compound with six carbon atoms, fourteen hydrogen atoms, and one oxygen atom. The first part of the name, 4-methyl, indicates that there is a methyl group (\(CH_3\)) attached to the fourth carbon atom in the chain. Pentan-2-ol tells us that there are five carbon atoms in the chain and that the hydroxyl group (OH) is attached to the second carbon atom. Therefore, the structural formula of 4-methyl pentane-2-ol can be written as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\). This can be further simplified as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\)which represents the complete structural formula of 4-methyl pentan-2-ol.4-methyl pentane-2-oil is an organic compound with a wide range of applications, including as a solvent, in the manufacture of cosmetics and perfumes, and as a flavoring agent in food and beverages. Its unique structure and properties make it a valuable component in various chemical and industrial processes.For more questions on methyl group
https://brainly.com/question/31238796
#SPJ8
How much phosphorous is in plant food?
Answers
The exact amount of phosphorous in plant food depends on the specific product, as well as the intended use and type of plant it is formulated for.
Plant food typically contains varying amounts of phosphorous, one of the three primary macronutrients essential for plant growth and health along with nitrogen and potassium.
For example, standard all-purpose plant food will often have a balanced ratio of the three macronutrients, including around 10% phosphorous. However, specific plant food products may have higher or lower amounts of phosphorous, with some formulated for high-phosphorus crops like tomatoes or flowering plants.
It's important to note that too much phosphorous can lead to imbalanced soil nutrient levels, potentially inhibiting the uptake of other essential micronutrients like iron and zinc. Therefore, it's important to follow label instructions and not over-fertilize with high-phosphorus plant food.
To know more about phosphorous click here:
https://brainly.com/question/28980790#
#SPJ11
true or false. a homogeneous catalyst can be in a different phase as the rest of the reaction as long as it is evenly distributed throughout the reaction mixture. true or false. a homogeneous catalyst can be in a different phase as the rest of the reaction as long as it is evenly distributed throughout the reaction mixture. true false
Answers
True , A catalyst is a substance that quickens a chemical reaction; therefore, in homogeneous catalysis, the catalyst and reactants are in the same phase. Most of the time, everything will exist as a gas or be contained within a single liquid phase.
Are there distinct phases for reactants and homogenous catalysts?The difference between heterogeneous and homogeneous catalysts is that the former are in the same phase as the latter are not.
Is there a single phase in a homogenous system?A single phase is what is meant by the notion of a pure substance or homogenous mixture. There are two or more phases in a heterogeneous mixture. Water and oil do not mix uniformly when combined; instead, two distinct layers are created.
To know more about homogeneous catalysis visit :-
https://brainly.com/question/20215227
#SPJ4
How many protons, neutrons, and electrons are in an atom of beryllium with a mass number of 9?
A) 5 protons,5 electrons,4 neutrons
B) 4 protons,4 electrons,4 neutrons
C) 4 protons,4 electrons,9 neutrons
D) 4 protons,4 electrons,5 neutrons
Answers
Answer:
c / a
correct me if im wrong
Answer:
D) 4 protons, 4 electrons, 5 neutrons
It was found that 3.0 g of M reacts with 1.0 g of oxygen to form the compound MO3. What is the atomic weight of M?
Answers
Answer:
144
Explanation:
The balanced equation would be :
M + 3 O ===> MO3
1 g of oxygen is 1/16 of a mole
It takes 1/3 as many moles of M to complete the reaction , or 1/48 mole
and 1/48 mole is 3 gm so atomic weight is 3 (48) = 144 u
You have been shipwrecked on a deserted island with no running water/fresh water, you have your clothes and a plastic bag containing your phone out of
charge and some sunscreen) How are you going to survive and escape?
Answers
Answer:
i have no clue
Explanation:
if two molecules of glucose (c6h12o6) are joined via condensation synthesis, the resulting molecule would have a molecular formula of .
Answers
The condensation reaction between two molecules of glucose would result in the formation of maltose (C12H22O11) and the release of water.
Carbohydrates are organic compounds that are used to store energy. An example of a carbohydrate is glucose, which is a single sugar or a monosaccharide. The process of forming larger molecules from the combination of simple sugars is called the condensation process. A water molecule is released in the process of combining the molecules together through a glycosidic bond.
The condensation reaction for two molecules of glucose (C6H12O6) results in the formation of maltose, a disaccharide, and the release of water. The molecular formula of maltose is C12H22O11. It can be observed that the condensation reaction shown below is balanced.
Glucose (C6H12O6) + Glucose (C6H12O6) → Maltose (C12H22O11) +H2O
To learn more regarding the condensation reaction of sugars, please refer to https://brainly.com/question/24950735.
#SPJ4
What would a liquid at 50 degrees Celsius. I want to know it’s melting and boiling point.
Answers
A liquid at 50 degrees Celsius would have a melting point of 50 degrees Celsius and a boiling point of approximately 173.15 degrees Celsius.
What is the liquid?
iquid is a state of matter that has a definite volume, but does not have a fixed shape. It is one of the four fundamental states of matter, along with solid, gas, and plasma. Liquids tend to have a greater volume than solids, but geneLrally take the shape of their container. Examples of liquids include water, milk, blood, gasoline, and oil. Liquids are composed of molecules that tend to have greater mobility and can easily flow past each other. Depending on the temperature, pressure, and composition of the liquid, it can undergo changes in the state of matter.
To learn more about liquid
https://brainly.com/question/225975
#SPJ1
Record all observations you made during the experiment. This should include if all of the benzophenone was transferred into the tube. Was anything unusual noticed during the melting? was all of the unknown added to the tube? was any stuck on the wire or thermometer? did all of the unknown melt? were there problems with mixing? did anything unusual happen - darkening, bubbling - during the melting of the unknown?
Answers
Observations made during the experiment include checking if all of the benzophenone was transferred into the tube, noting any unusual occurrences during the melting process,
ensuring that all of the unknown was added to the tube and not stuck on the wire or thermometer, monitoring the melting of the unknown, checking for problems with mixing, and noting any unusual darkening or bubbling that occurred during the melting of the unknown.
Scientific experiments must include observations because they help researchers gather information and spot patterns or trends in their findings. The experiment in this instance was centred on observing how the benzophenone and the unknown material behaved while being heated and melted in a test tube. The melting points, solubility, and chemical reactivity of these substances, as well as other characteristics, can all be learned from these observations. In order to interpret their findings and make judgements regarding the behaviour of the substances under study, researchers must meticulously record their observations. They can then utilise this knowledge to improve their experiments or come up with fresh theories regarding the characteristics of matter.
Learn more about observations here:
https://brainly.com/question/28041973
#SPJ4
a. the results show that the initial rate increased when the initial concentration of either reactant increased. explain why increasing the concentration or partial pressure of a reactant can increase the rate at which the reaction proceeds. b. what environmental factor could be altered in order to increase the initial reaction rate in experiment one if the initial concentrations of the reactants remained the same? justify your answer. c. what is the order of the reaction with respect to no? show your work. d. what is the order of the reaction with respect to o2? show your work. e. find the rate law for this reaction. f. what is the overall order of this reaction? g. find the rate constant, k, for the reaction. (include the units) h. if the same three experiments were conducted at a lower temperature, would the magnitude of the rate constant be greater than, less than, or equal to the value calculated above? justify your answer.
Answers
a) Increasing the concentration or partial pressure of a reactant can increase the rate at which the reaction proceeds because there are more reactant molecules present to participate in the reaction.
b) Increasing the temperature would increase the kinetic energy of the reactant molecules, which would lead to more successful collisions and a higher reaction rate.
c) The initial rate doubled when the concentration of NO doubled, the reaction is first order concerning NO.
d) The initial rate of the reaction in experiment one is 0.40 M/s, and the initial rate of the reaction in experiment three is 0.20 M/s.
e) The rate law for this reaction can be written as: rate = k[NO][O2].
f) The overall order of this reaction is the sum of the orders concerning each reactant, which is 1 + 1 = 2.
g) The rate constant, k, for the reaction can be calculated using the formula: k = (rate/\([NO]^{2}[O2]\)).
h) The magnitude of the rate constant would be less than the value.
a. Increasing the concentration or partial pressure of a reactant can increase the rate at which the reaction proceeds because there are more reactant molecules present to participate in the reaction. This means that there is a higher probability of successful collisions between reactant molecules, leading to a higher reaction rate.
b. One environmental factor that could be altered to increase the initial reaction rate in experiment one if the initial concentrations of the reactants remained the same is the temperature.
c. To find the order of the reaction concerning NO, we need to compare the initial rates of the reactions in experiments one and two. The initial rate of the reaction in experiment one is 0.40 M/s, and the initial rate of the reaction in experiment two is 0.80 M/s. Since the initial rate doubled when the concentration of NO doubled, the reaction is first order concerning NO.
d. To find the order of the reaction concerning O2, we need to compare the initial rates of the reactions in experiments one and three. The initial rate of the reaction in experiment one is 0.40 M/s, and the initial rate of the reaction in experiment three is 0.20 M/s. Since the initial rate decreased by half when the concentration of O2 doubled, the reaction is first order concerning O2.
e. The rate law for this reaction can be written as: rate = k[NO][O2].
f. The overall order of this reaction is the sum of the orders concerning each reactant, which is 1 + 1 = 2.
g. The rate constant, k, for the reaction can be calculated using the formula: k = (rate/[NO]²[O2]) where the rate is the rate of the reaction, [NO] is the concentration of NO, and [O2] is the concentration of O2. The units of k will depend on the units of rate and the concentrations of the reactants.
h. If the same three experiments were conducted at a lower temperature, the magnitude of the rate constant would be less than the value.
Learn more about the chemical reactions at
https://brainly.com/question/14859519?referrer=searchResults
#SPJ4
A substance made up of only one kind of atom is called
Answers
A substance made up of only one kind of atom is called Elements.
An element is a pure substance made up of only one kind of atom. A compound is substance made up some of more than one element. in some of the elements atoms are joined in groups of two or more called as molecule . example : oxygen is an element made up of only one kind of atom that is oxygen atoms. there are 118 known elements , and are represented on the periodic table.
Thus, A substance made up of only one kind of atom is called Elements.
To learn more elements here
https://brainly.com/question/8396323
#SPJ1
Find the lattice energy of MgH2(), how with a erie of tep when the Enthalpie of formation for calcium hydride i given a; (ΔHf = −75. 3 kJ/mol for MgH2)
Answers
the lattice energy of MgH2() is 2791 kJ/mol with a series of step when the Enthalpies of formation for calcium hydrided is given.
Do energies journal free?An article publishing charge (APC) is required of authors or research funders to cover publication costs in open access journals that do not charge subscription fees. This guarantees that everyone will be able to access your article promptly and without charge in the future.
Solution:
the lattice energy of MgH2() is :
ΔHf(−75. 3 kJ/mol ) =S+0.5D+IE+EA+U
ΔHf(−75. 3 kJ/mol )= 2791 kJ/mol
How good are MDPI journals?The papers published in MDPI Special Issues are of extremely high quality! When I published my research with MDPI (Energies Journal), it was a wonderful experience. Both the submission of the manuscript and the timing of publishing were excellent.
To know more about energy visit
brainly.com/question/11399976
#SPJ4