Suppose two students grab an end of a rope. Both shake the rope once,
but one makes a crest and the other a trough traveling at each other. What would
you see when the crest and trough meet? What type of interference does this show?
Answers
When the crest and trough of two waves meet, they undergo destructive interference, causing the amplitude of the resulting wave to be smaller than that of either individual wave.
In this scenario, the two students shaking the rope create waves that travel toward each other. One student creates a crest, which is a point of maximum positive displacement, while the other creates a trough, which is a point of maximum negative displacement. When these two points meet, they interfere destructively, resulting in a wave with a smaller amplitude than either individual wave.
This phenomenon of destructive interference is a result of the superposition principle of waves, which states that the displacement of two waves at any point in space and time is the algebraic sum of the individual displacements of the waves.
When two waves of equal amplitude and opposite phase meet, they cancel each other out, resulting in a wave with a smaller amplitude or even no wave at all.
To know more about destructive interference, refer here:
https://brainly.com/question/31857527#
#SPJ11
Related Questions
Zirconium (Zr) has an average atomic mass of 91.22 amu and is made up of the isotopes Z90r, Z91r, Z92r, Z94r, and Z96r. The atom of which isotope has the greatest mass
Answers
Answer:
96 Zr
Explanation:
just took the quiz and that was the right answer
WILL MARK BRAINLIEST PLS HELP! Predict whether or not the following single replacement reaction will happen. If the reaction will occur, write a complete balanced equation.
Bromine + Sodium chloride →
Answers
The single replacement reaction between bromine (Br₂) and sodium chloride (NaCl) will occur.
Bromine is a more reactive halogen than chlorine, and it can displace chlorine from its compound. The balanced chemical equation for the reaction is:
Br₂ + 2NaCl → 2NaBr + Cl₂
In this reaction, bromine replaces chlorine to form sodium bromide and chlorine gas. The reaction occurs because bromine is more reactive than chlorine, and it has a higher tendency to gain an electron to form a bromide ion.
Sodium chloride contains positively charged sodium ions and negatively charged chloride ions. When bromine is added to sodium chloride, it reacts with chloride ions to form sodium bromide and chlorine gas. Sodium bromide is an ionic compound that contains positively charged sodium ions and negatively charged bromide ions.
Chlorine gas is a diatomic molecule that contains two chlorine atoms covalently bonded together.
To know more about replacement reaction, visit :
https://brainly.com/question/15995721
#SPJ1
One substance flows down its concentration gradient, and the energy is harnessed to move another substance up its gradient in the same direction. This best describes.
Answers
When a substance moves down its concentration gradient, and energy is used to move another substance up its concentration gradient in the same direction is antiport secondary active transport
The primary active transport establishes the Na+ or H+ gradient. So to move substances against their concentration gradient, secondary transport uses energy derived from the hydrolysis of Adenosine triphosphate (ATP).
Secondary active transport is of two types - symport and antiport. When two or more substances move in the opposite direction using energy, it is termed an antiport. In this, one substance move inside, and another substance moves out of the cell. When two or more substance move in the same direction using energy is called symport. In this, either they are moving inside or out of the cell but move in the same direction.
Therefore, when a substance moves down its concentration gradient, and energy is used to move another substance up its concentration gradient in the same direction is antiport secondary active transport.
To know more about active transport, refer to the below link:
https://brainly.com/question/12133248
#SPJ4
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
Which scenario describes a relationship of parasitism? Responses: A. A tick attaches itself to the skin of a dog. B. A wolf catches a rabbit for a meal. C. Birds feed off the insects that are stirred up from the grasses as cattle move through. D. Oxpecker birds eat parasitic ticks off the backs of zebras.
Please hurry
Answers
A tick attaches itself to the skin of a dog describes a relationship of parasitism.
The relationship between two species of plants or animals known as "parasitism" occurs when one species gains an advantage over the other, sometimes without really harming the host organism. the tick's life cycle The tick's life cycle is Ixodes scapularis. Malaria life cycle caused by Ixodes scapularis
Either endoparasites, which may either be intercellular (inhabiting spaces in the host's body) or intracellular (inhabiting cells in the host's body), or ectoparasites, which live on the body surface of the host and do not usually cause disease in the host, include ticks, fleas, leeches, and lice. A third organism known as the carrier, or vector, is frequently used by intracellular parasites, such as bacteria or viruses, to spread to the host.
To know more about parasitism visit :https://brainly.com/question/22589174
#SPJ1
List 1 advantage and 1 disadvantage of a series circuit.
Please help!!!

Answers
Answer:
The advantage is that, you can add additional power devices usually using batteries.The disadvantage is ... if one component in a series circuit fails, then all the components in the circuit fail because the circuit has been broken.What would be the advantages of using Al(OH)3 as an antacid rather than NaHCO3, which undergoes the following reaction with stomach acid?
NaHCO3(aq) + HCl(aq) →→→→ NaCl(aq) +
H₂O(l) + CO₂(g)
Answers
Answer:
Aluminum hydroxide (Al(OH)3) has several advantages over sodium bicarbonate (NaHCO3) as an antacid. One advantage is that Al(OH)3 does not neutralize stomach acid as quickly as NaHCO3 does, so it can provide longer-lasting relief for symptoms of heartburn and indigestion. Additionally, Al(OH)3 is less likely to cause side effects such as diarrhea, which can be a common problem with overuse of NaHCO3. Furthermore, Al(OH)3 can also adsorb excess stomach acid and form a physical barrier that helps to protect the stomach lining.
Reptiles, birds, and mammals reproduce by what type of
fertilization?
The correct answer is Internal
Answers
Internal Fertilization
ASAP please and Thankyou

Answers
Specific heat is used to explain why different substances what?
Answers
Specific heat is used to explain why different substances changes the temperature at different rate.
What is temperature?Temperature is a physical quantity which is expressed as quantitatively the perceptions of the coldness and hotness. Temperature is measured with a thermometer.
What is specific heat?It is defined as the amount of heat which is used to require to increase the temperature by 1°C.
The SI unit thorough which it is measured is J/ g/ °C.
Let's take an example of specific heat of water which is 4.184J/ g/ °C.
If the specific heat capacity of any substance is observes to be high, it will take more time for heating or cooling. If the specific heat capacity of any substance is observes to be high, it will take more time for heating or cooling.Therefore, specific heat is used to explain why different substances changes the temperature at different rate.
learn more about special heat:
https://brainly.com/question/28852989
#SPJ1
photoelectron spectroscopy is used to remove one electron from an atom or molecule. this process was used to remove one electron from potassium. how many different ionization energy bands were found? (ignore effects from spin).
Answers
One can observe two ionization energy bands in the photoelectron spectra of potassium.
The number of ionization energy bands that can be found in photoelectron spectroscopy depends on the specific atom or molecule being studied, as well as the conditions under which the spectroscopy is performed.
For potassium, the most common form of photoelectron spectroscopy is X-ray photoelectron spectroscopy (XPS), which typically results in the observation of two ionization energy bands one corresponding to the removal of an electron from the 1s orbital and another corresponding to the removal of an electron from one of the higher-energy orbitals, such as the 2p or 2s orbitals.
It's worth noting that the number of ionization energy bands observed can be influenced by various factors, such as the level of energy resolution of the spectrometer and the presence of impurities or other chemical species in the sample. Additionally, the presence of spin-orbit coupling can give rise to additional, splitted peaks in the XPS spectra, which would increase the number of ionization energy bands observed.
To learn more about Ionization energy :
https://brainly.com/question/15702918
#SPJ4
What is in the solar system?
A. All the above
B. asteroids and comets
C. The sun and everything thing that orbits around it
D. planets and their moons
PLEASE HELP
Answers
Answer:
all of the above
Explanation:
all of this is in the solar system
2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl, silver chloride, are produced from 5.0 g of AgNO3, silver nitrate?
Answers
4 g AgCl
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Stoichiometry
Reading a Periodic TableUsing Dimensional AnalysisExplanation:Step 1: Define
[RxN] 2AgNO₃ + BaCl₂ → 2AgCl + Ba(NO₃)₂
[Given] 5.0 g AgNO₃
Step 2: Identify Conversions
[Reaction - Stoich] 2AgNO₃ → 2AgCl
Molar Mass of Ag - 107.87 g/mol
Molar Mass of N - 14.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of Cl - 35.45 g/mol
Molar Mass of AgNO₃ - 107.87 + 14.01 + 3(16.00) = 169.88 g/mol
Molar Mass of AgCl - 107.87 + 35.45 = 143.32 g/mol
Step 3: Stoichiometry
Set up: \(\displaystyle 5.0 \ g \ AgNO_3(\frac{1 \ mol \ AgNO_3}{169.88 \ g \ AgNO_3})(\frac{2 \ mol \ AgCl}{2 \ mol \ AgNO_3})(\frac{143.22 \ g \ AgCl}{1 \ mol \ AgCl})\)Multiply/Divide: \(\displaystyle 4.21533 \ g \ AgCl\)Step 4: Check
Follow sig fig rules and round. We are given 1 sig fig.
4.21533 g AgCl ≈ 4 g AgCl
a particular protein sample is found to have 1 atom of iron in its chemical formula. if the mass percent of fe in the compound is 0.330% fe, what is the molar mass of the protein?
Answers
The molar mass of the protein is 16,922.73 g/mol.
Given:
A protein sample, with 1 atom of iron in its chemical formula.
The mass percent of iron in the protein is 0.330%.
To find:
The molar mass of protein
Solution:
The mass percentage of iron in protein = 0.330%
The atomic mass of iron = 55.845 g/mol
Number of iron atoms in protein = 1
The molar mass of protein = M
The mass percentage of elements in a compound is given by:
\(=\frac{\text{Atomic mass of element}\times \text{Number of atoms of element}}{\text{Molar mass of compound}}\times 100\)
The percentage of iron in the protein will :
\(0.330\%=\frac{55.845g/mol\times 1}{M}\times 100\\\\M=\frac{55.845g/mol\times 1}{0.330}\times 100\\\\=16,922.73 g/mol\)
The molar mass of the protein is 16,922.73 g/mol.
Learn more about the mass percentage of elements here:
brainly.com/question/12247957?referrer=searchResults
brainly.com/question/419592?referrer=searchResults
A student investigates the reaction between sodium hydroxide solution and dilute sulfuric acid.
He does a titration to find the concentration of the sulfuric acid.
(a) Give the colour change of the methyl orange indicator at the end-point.
Answers
Explanation:
the colour change will be from orange to pink
The color change of the methyl orange indicator at the end-point will show orange to pink.
What is indicator?In an acidic or basic medium, an indicator is just a material that changes color. Indicators are termed indicators because they show one color in an acidic media and different colors in a basic medium. Indicators come in a variety of shapes and sizes: Natural indicators are indicators acquired from natural sources.
It is a just a acid base reaction in which methyl orange acts as a indicator.
In this reaction, when sodium hydroxide solution and dilute sulfuric acid are reacts with each other then after using( indicator)methyl orange the color will be change form orange to pink which indicates the nature of solution will be acidic.
To know more about indicator.
https://brainly.com/question/12489874.
#SPJ2
Please Help!!!
You placed 6.35 g of a mixture containing unknown amounts of BaO(s) and MgO(s) in a 3.50-L flask containing CO₂(g) at 30.0°C and 750. torr. The solid BaO and MgO in the flask completely reacted to form BaCO₃(s) and MgCO₃(s), respectively. After the reactions to form BaCO₃(s) and MgCO₃(s) were completed, the pressure of CO₂(g) remaining was 115 torr, still at 30.0°C. Calculate the mass of BaO(s) in the initial mixture in grams. (Assume ideal gas behavior).
Answers
By the use of stoichiometry, the mass of BaO in mixture is 0.06475 g.
What is the mass of BaO?From the question;
V = 3.50 LT = 303 K P = 750 torrMass of BaO in the mixture = x grams
Mass of MgO in the mixture = (6.35 - x) grams
Number of moles of CO2 initially present;
PV = nRT
= ((750/760) × 3.50) / 0.0821 × 303
n= 0.139
Number of moles of CO2 at the end;
n= PV /RT
= ((245/760) × 3.5) / 303 × 0.0821
= 0.045 mole
Amount of CO2 reacted;
= 0.139 - 0.045
= 0.044 mole
Now;
amount of reacted CO2 = ( amount of BaO + amount of MgO)
amount of BaO in mixture = x / 153 mole
amount of MgO in mixture = 6.35 - x mole / 40
Hence;
= x/ 153 + 6.35/40 = 0.094
= x/153 + 6.35 / 40 - x/40 = 0.094
= x (1/40 - 1153) = (6.35/40 - 0.094)
= x × 10.018464
= 0.06475 g
Hence, the mass of BaO in mixture is 0.06475 g.
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1
How did Robert Hooke contribute to the cell theory?...

Answers
calculate the ph and pOH when pH is 2.37, 11.05, or 6.5
Answers
: When pH is 2.37, pOH can be calculated using the formula pH + pOH = 14. Therefore, pOH = 14 - 2.37 = 11.63.
Similarly, when the pH is 11.05, the pOH can be calculated as pOH = 14 - 11.05 = 2.95. Finally, when the pH is 6.5, the pOH can be calculated as pOH = 14 - 6.5 = 7.5.
Learn more about pOH at:
https://brainly.com/question/17144456
#SPJ1
provide the structure for 5-chloro-2-propyl-1-heptanol.
Answers
The structure for 5-chloro-2-propyl-1-heptanol can be drawn by drawing a 7 carbon chain with (OH) functional group on C1, adding propyl group on C2 and Cl on C5
5-Chloro-2-propyl-1-heptanol is an organic compound that belongs to the class of alcohols.
The IUPAC name of the compound is 5-chloro-2-propylheptan-1-ol, which denotes the position of the hydroxyl group on the carbon chain. The prefix "heptan-" refers to the seven-carbon chain, and the suffix "-ol" denotes the presence of an alcohol functional group (-OH).
5-Chloro-2-propyl-1-heptanol is a colorless to pale yellow liquid with a strong odor. It is a primary alcohol. It is a versatile chemical intermediate that can be used to prepare a variety of other compounds, including pharmaceuticals, agrochemicals, and flavors. It is also used as a solvent and a reagent in organic synthesis
Thus, the structure for 5-chloro-2-propyl-1-heptanol can be drawn by drawing a 7 carbon chain with (OH) functional group on C1, adding propyl group on C2 and Cl on C5
To learn more about IUPAC :
https://brainly.com/question/28872356
#SPJ11
2. Predict the shift in the reaction with each stress shift rt, shift left, or no
HEAT + Ti(s) + 2C1 (g)
a. CI, (g) is added to the system.
b. TiCk (g) is removed from the system.
TiCI (g)
c. The temperature of the container is decreased.
d. The pressure of the container is increased.,
e. Ti(s) is added to the system.
Answers
b. The removal of TiCl(k) from the system will shift the reaction right, towards the products, to counteract the decrease in the concentration of TiCl(k).
c. Decreasing the temperature of the container will shift the reaction to the right, towards the products, to counteract the decrease in kinetic energy of the system.
d. Increasing the pressure of the container will shift the reaction to the left, towards the reactants, to counteract the increase in pressure caused by the decrease in volume.
e. Adding Ti(s) to the system will not cause a shift in the reaction because Ti(s) is not involved in the reaction.
A compound has an empirical formula of
CH20. What is its molecular formula, if its
molar mass is 180 g/mol?
(C=12.01 amu, H=1.008 amu, O=16.00 amu)
Answers
Answer:
C6H12O6
Explanation:
Molar mass of empirical formula:
12.01+2*1.008+16=30.026
Divide molar mass of molecular formula by 30.026
180/30=~6
Scale up the empirical formula by a factor of 6.
C6H12O2 (glucose)
Answer:
C6H12O2
Explanation:
Scale up the empirical formula by a factor of 6.
C6H12O2 (glucose)
A graduated cylinder contains 35.78 mL of water an object is placed in the cylinder volume of water rises to 41.23 mL what is the volume of the object
Answers
Answer:
5.45 mL
Explanation:
Archimedes' principle tells us that the volume of water an object displaces when it is submerged is equal to the object's volume.
This means that in order to calculate the object's volume, we can calculate the amount of water it displaced:
41.23 mL - 35.78 mL = 5.45 mLThus the volume of the object is 5.45 mL.
explain how the principle of faunal succession connects prehistoric life to the age of sedimentary rocks
Answers
The principle of faunal succession is a key concept in the study of prehistoric life and the age of sedimentary rocks. It is based on the idea that the fossilized remains of different species of animals and plants found in sedimentary rocks can be used to infer the relative age of the rocks and the conditions that existed when they were formed.
What are sedimentary rocks?The principle of faunal succession states that over time, the species of animals and plants that lived in a particular area changed and evolved. As a result, the fossilized remains of different species found in sedimentary rocks can be used to infer the relative age of the rocks.
This principle is based on the idea that the fossil record is a record of the evolution of life on Earth. In addition, the principle of faunal succession also provides insight into the ancient environment and climate. For example, the presence of certain types of fossils, such as coral reefs or tropical plants, may indicate that the area was once a warm, shallow sea.
In all, the principle of faunal succession is a powerful tool that connects prehistoric life to the age of sedimentary rocks. By studying the fossilized remains of different species found in sedimentary rocks, scientists can infer the relative age of the rocks and the conditions that existed when they were formed.
Learn more about sedimentary rocks from
https://brainly.com/question/7437433
#SPJ1
where are the impurities located after the recrystallization?
Answers
The impure substance will crystallize in a purer form because the impurities won't crystallize eventually, therefore leaving the impurities behind in the solution.
Recrystallization is a considerably crucial technique for purifying nonvolatile organic solids. Recrystallization implicates dissolving the material to be purified in a relevant hot solvent. As the solvent cools, the solution evolves saturated with the solute and the solute crystallizes out. The compound is dissolved in the solvent, the solution is purified to deduct the insoluble impurities, and the solvent is evaporated to create the solid compound. The insoluble impurities are left behind in the filter paper.
To learn more about recrystallization visit here:
https://brainly.com/question/30701380
#SPJ4
A comet has an aphelion distance of 34 A.U. and an orbital period of 91 years. Calculate the perihelion
Answers
The perihelion distance of the comet is approximately 19.36 A.U., based on the given aphelion distance of 34 A.U. and orbital period of 91 years, using Kepler's laws of planetary motion.
To calculate the perihelion distance of the comet, we can make use of Kepler's laws of planetary motion and the relationship between the aphelion and perihelion distances.
Kepler's laws state that the square of the orbital period (T) is proportional to the cube of the average distance between the comet and the sun (r).
T^2 ∝ r^3
We are given that the orbital period (T) is 91 years and the aphelion distance (r) is 34 astronomical units (A.U.). Let's represent the perihelion distance as p.
Since the ratio of the squares of the periods is equal to the ratio of the cubes of the distances, we can set up the following equation:
(T_aphelion^2 / T_perihelion^2) = (r_aphelion^3 / r_perihelion^3)
Substituting the given values:
(91^2 / T_perihelion^2) = (34^3 / p^3)
We can solve for p by rearranging the equation:
p^3 = (34^3 * T_perihelion^2) / 91^2
Taking the cube root of both sides:
p = (34 * T_perihelion)^(2/3) / 91^(2/3)
Substituting the value of the orbital period (T_perihelion = 91 years):
p = (34 * 91)^(2/3) / 91^(2/3)
Calculating this expression, we find:
p ≈ 19.36 A.U.
Therefore, the perihelion distance of the comet is approximately 19.36 astronomical units.
To read more about comet, visit:
https://brainly.com/question/31893459
#SPJ11
How does the law of conservation of mass tell you that reacting zinc with hydrochloric acid can never produce aluminum oxide?
Answers
The law of conservation of mass explains clearly to me that a reaction between zinc and hydrochloric acid can never produce aluminum oxide as the product formed was not part of the reaction.
What is it meant by the law of conservation of mass?The law of conservation of mass or matter state matter is neither created not destroyed but changes from one form to another. Applying this to the task above, when zinc combine with hydrochloric acid, it forms zinc chloride and librate hydrogen gas.
The chemical equation is given below:
Zn (s)+2HCl(aq) → ZnCl₂(g) H₂O
So therefore, we can confirm that matter can neither be created not destroyed in a chemical reaction.
Read more on the law of conservation of mass:
https://brainly.com/question/15289631
#SPJ1
Matter cannot change liquid to solid true or false
Answers
Answer:
yes, I am pretty sure it can
1.From the pictures above,which shows a higher temperature?why?
2.which has more heat?why?
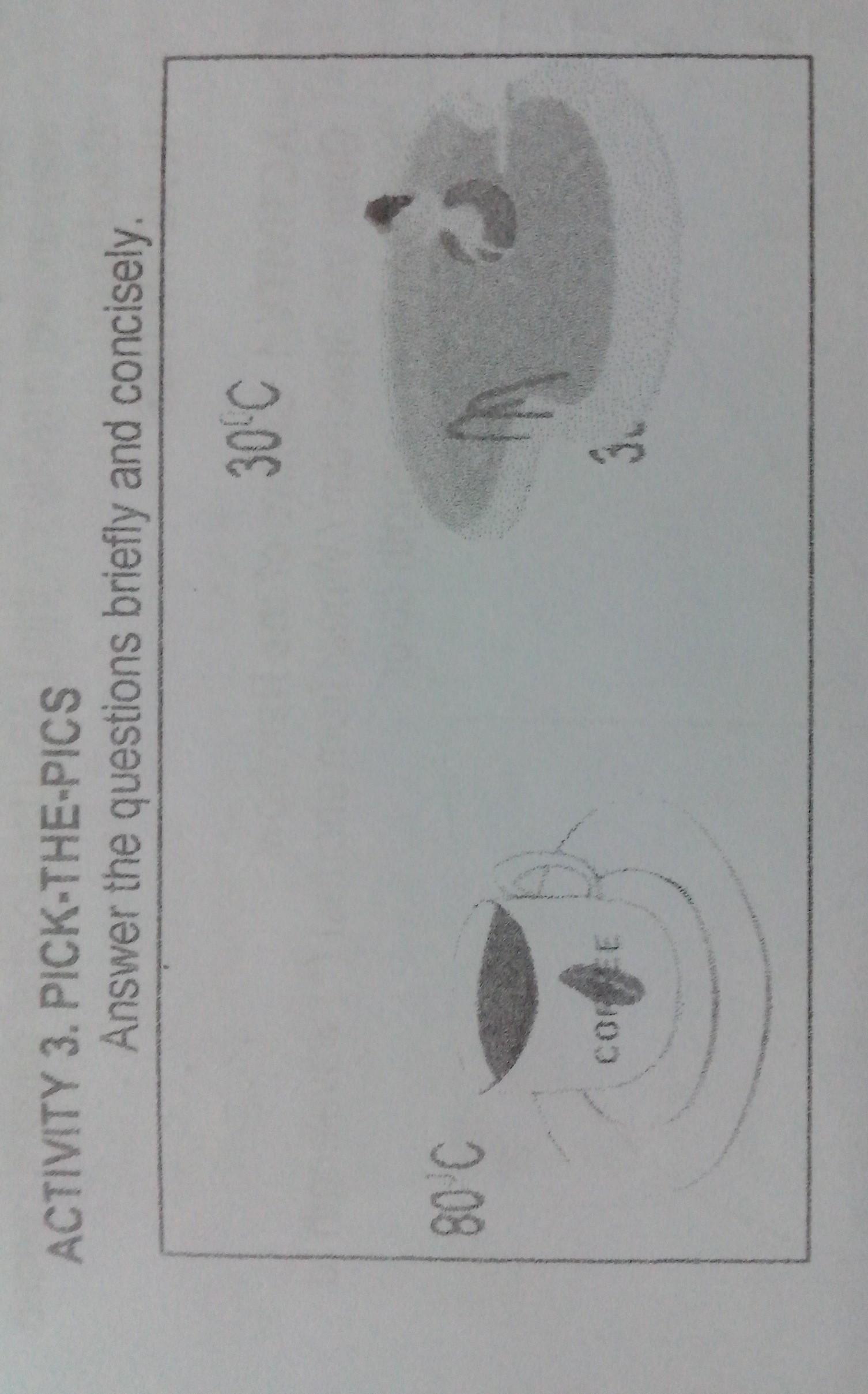
Answers
Answer:
The coffee has a higher temperature because a pool is so you can have fun and relax in while coffee can warm you up by drinking it.
Explanation:
Hope it helps :)
The type of substance has a pH lower than 7. They turn litmus paper red. They are sour and sharp-tasting. They react with metals. This type of substance is a...
1. Acid
2.Base
3. Neutral substance
Please help lol
Answers
The answer is Acid
Explanation:
It's acid, because from its definition we can say that acid turns blue litmus paper red, and it is also corrosive and has a sour taste.
Coming to the pH scale, which is a measure of the acidity and alkalinity of s substance.It is said to be acidic between the range of 1 - 6.9 and neutral at 7, and alkaline between the range of 7.1 - 14Which of the following alkyl halides could be successfully used to form a Grignard reagent? 1. OHCH2CH2CH2CH2Br 2. H2NCH2CH2CH2Br 3. BrCH2CH2CH2COOH 4. CH3N(CH3)CH2CH2Br
Answers
Out of the given alkyl halides, the fourth option, CH3N(CH3)CH2CH2Br, could be successfully used to form a Grignard reagent. This is because Grignard reagents are formed by the reaction of an alkyl halide with magnesium metal in dry ether.
The resulting product is an organomagnesium compound, which can be further used in organic synthesis. In option 1, the hydroxyl group would interfere with the reaction, while in option 2, the amino group would also hinder the reaction. Option 3 has a carboxylic acid group, which is not an alkyl halide. Therefore, option 4 is the only suitable alkyl halide for forming a Grignard reagent.
A Grignard reagent is formed by reacting an alkyl halide with magnesium in an ether solvent. To be successful, the alkyl halide must not contain any acidic protons (H) that can react with the Grignard reagent. In the given options, 1. OHCH2CH2CH2CH2Br and 3. BrCH2CH2CH2COOH have acidic protons in their alcohol and carboxylic acid groups, respectively, and thus cannot form a Grignard reagent. 2. H2NCH2CH2CH2Br has an acidic proton in the amine group and is also unsuitable. The only suitable option is 4. CH3N(CH3)CH2CH2Br, as it lacks any acidic protons and can successfully form a Grignard reagent.
To know about halides:
https://brainly.com/question/8486709
#SPJ11