the examples for anions with names charges and chemical symbols
Answers
eg no 1= Calcium
charge=> 2+
symbol Ca
eg no 2= Hydroxide
charge 1-
symbol=>OH
sorry I only know 2 eg
Related Questions
What is the net ionic equation for the following reactant?
Al (s) + HCl (aq) -->
Answers
Explanation:
hope the picture above helps u to understand:)

Which option lists the layers of the rainforest in the correct order from top to bottom?
emergent layer, canopy, forest floor, shrub layer
emergent layer, canopy, understory, forest floor
forest floor, emergent layer, canopy, understory
forest floor, understory, canopy, emergent layer
Answers
Answer: B.
Explanation:
Trust me already took the quiz on it its B.
Answer:
Yes its B
Explanation:
PLS HELP URGENT
Electron dot diagrams
Use your periodic table to write the electron dot diagrams for the following atoms.
1. Calcium (Ca)
2. Polonium (Po)
3. Moscovium (Mc)
4. Boron (B)
5. Fluorine (F)

Answers
Sodium chloride (nacl) is the chemical name for table salt and potassium chloride (kcl) is a common salt substitute. Using the periodic table, choose the correct statement about how these compounds form.
Answers
Sodium and potassium lose electrons and chlorine gains them.
What is electrons?Electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.\The electron was discovered in 1897 by the English physicist J.J. Thomson during investigations of cathode rays. His discovery of electrons, which he initially called corpuscles, played a pivotal role in revolutionizing knowledge of atomic structure. Under ordinary conditions electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus.To learn more about nucleus refer to:
https://brainly.com/question/141626
#SPJ4
write out the balanced equation for the reaction of 1 mole of naoh with 1 mole of h3po4
Answers
The balanced equation for the reaction of 1 mole of NaOH with 1 mole of \(H_{3}PO_{4}\) can be represented as follows:
3NaOH + \(H_{3}PO_{4}\) → \(Na_{3}PO_{4}\) + 3\(H_{2}O\)
In this reaction, sodium hydroxide (NaOH) reacts with phosphoric acid (\(H_{3}PO_{4}\)) to form sodium phosphate (\(Na_{3}PO_{4}\)) and water (\(H_{2}O\)).
To balance the equation, it is necessary to ensure that the number of atoms of each element is equal on both sides of the reaction.
In this case, we need 3 moles of NaOH to react with 1 mole of \(H_{3}PO_{4}\) to form 1 mole of \(Na_{3}PO_{4}\) and 3 moles of \(H_{2}O\). This balanced equation accurately represents the stoichiometry of the reaction between NaOH and \(H_{3}PO_{4}\).
To know more about phosphoric acid, refer here:
https://brainly.com/question/29344496#
#SPJ11
Which missing item would complete this beta decay reaction?

Answers
Answer:
The answer is option A.Hope this helps you
if the percent yield is 80.2%, what mass of k (in grams) is needed to obtain 27.6 g of h2? (assume in excess of hcl).
Answers
Stoichiometry and the idea of percent yield can be used to calculate the mass of K required to produce 27.6 g of H2. The reaction's balanced chemical equation is 2 K + 2 HCl 2 KCl + H2.
How much kclo3 is required to make 32.0 g of o2?Response and justification Hence, 126.23 g of potassium chlorate are needed to generate 32 g of oxygen.
How is yield determined in g?Divide the mass of the reactant by the molecular weight to get the mass per mole. As an alternative, we can multiply the liquid solution's density in grammes per millilitre by the amount of reactant solution in millilitres. Next, divide the outcome by the reactant's molar mass.
To know more about Stoichiometry visit:-
brainly.com/question/30215297
#SPJ1
Use the information in the square to answer the questions about copper. A purple box has C u at the center and 29 above. Below it says copper and below that 63.55.How many protons are in an atom of copper
Answers
The number of protons in an atom of copper is 29.
It can be determined from the element symbol, Cu, which stands for Copper. The atomic number of an element is the number of protons in the nucleus of an atom of that element and is typically located above the element symbol. In this case, the atomic number of copper is 29, as indicated by the number 29 above the element symbol Cu. This is the information provided in the square.
Additionally, we can calculate the number of neutrons by the given information.
The number of neutrons= Mass number - Atomic number.
In this case,
The number of protons in Cu= 63 - 29 = 34.
Read more about Atomic Configuration:
https://brainly.com/question/14190064
#SPJ4
examples is potassium?
Answers
Answer: ... potassium citrate, potassium chloride, and potassium with dextrose and sodium chloride. Potassium atom is an alkali metal atom.
Explanation:
two molecules with the same structural formula must have:
Answers
Consider what happens when a sample of the explosive TNT is detonated under atmospheric pressure. What is the sign of q for this process?
Answers
The sign of q for this process is negative as heat is released into the surroundings when a sample of explosive TNT is detonated under atmospheric pressure.
When TNT(Trinitrotoluene) is detonated under atmospheric pressure, it undergoes an exothermic reaction which releases a large amount of heat and gas. This process is highly exothermic and releases energy in the form of heat, light, and a shock wave. The force exerted by the air on the surface above it is called Atmosphere Pressure. Atmospheric pressure is measured using a device called barometer. The standard atmosphere is a unit of pressure given by 101,325 Pa, which is equivalent to 1013.25 millibars, 760 mm Hg, 29.9212 inches Hg, or 14.696 psi.
Learn more about atmospheric pressure : https://brainly.com/question/30215097
#SPJ11
Name any three organic fertilizer using by farmers ?
Answers
Hope this helped :)
Answer:
Typical organic fertilizers include mineral sources, all animal waste including meat processing, manure, slurry, and guano, plant based fertilizers, such as compost, and biosolids.
As the number of protons increases but the distance between the protons and electron REMAINS THE SAME...
O the electronegativity stays the same.
O the electronegativity decreases.
O the electronegativity increases.
Answers
Answer:
The correct option is;
The electronegativity increases
Explanation:
The electronegativity is the measure of an atom's ability to attract a shared electron pair. The electronegativity of an atom is dependent on the atom's atomic number and the separation distance between the electrons in the valence shell and the positively charged nucleus such that an increase in the atomic number results in an increase in electronegativity and an increase in the distance between the valence electrons and the nucleus, leads to a decrease in electronegativity.
Which person do you agree with the most?
A Casey: The human population had been fairly constant throughout
Earth's history.
Allayna: Population size is increasing at a significant rate due to
advances in technology, medicine, and sanitation.
© Karl: Population size has been decreasing over time because people
are having fewer children.
Answers
Pls help me plsssss my mom is coming home soon I’ll mark brainliest hurrrrrrrrry

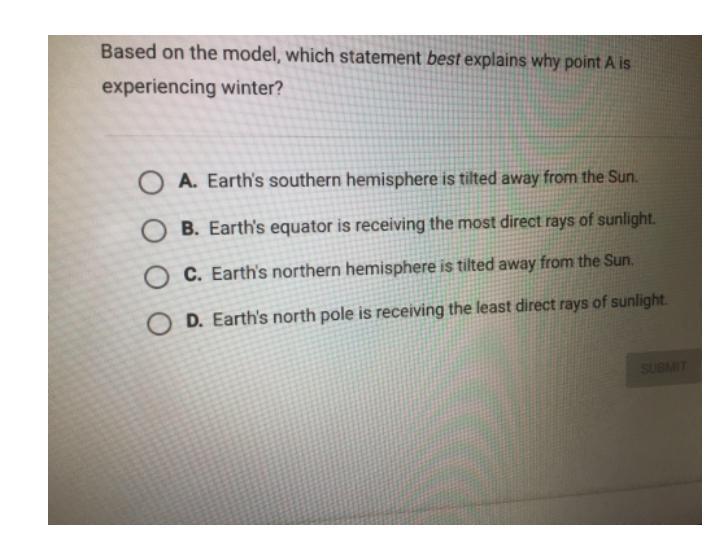
Answers
Answer:
Its A, sorry I wrote something else.
Explanation:
The equator is the warmest, and the sunlight is not pointing towards point A.\
Friendly guy that likes to help
the screenshts below





Answers
Answer:
yes so for example one
Explanation:
yeah so
Find the amount of heat in joules required to raise the temperature of 28.0 grams of water from 30.0°C to 60.0°C.
Answers
Answer: 3510 J
Explanation:
q = m x Cs x delta T
m = 28.0 g
Cs = 4.18 J/g x ⁰C
delta T = Final temperature - Initial temperature = 60.0⁰C - 30.0⁰C = 30.0⁰C
q = m x Cs x delta T
q = 28.0 g x (4.18 J/g x ⁰C) x 30.0⁰C = 3511.2 J
3510 J for the correct number of significant figures.
Grams and ⁰C cancel and you are left with Joules.
Which unit is used for measuring atomic mass?
Answers
Answer: Amu
Explanation:
If the empirical formula of a compound is CH2O what is a possible molecular formula for
the compound?
A C2H4O2
B CH20
C C5H705
D CH40
Answers
which of the following will cause a decrease un the percent ionization of H2SO4 when added to a solution of H2SO4 at equilibrium ?
a) SO3
b) Na2SO3
c) CaSO3
d) K2SO4
Answers
The addition of any substance that reacts with H+ ions (protons) will cause a decrease in the percent ionization of H2SO4, as this will shift the equilibrium towards the side with more undissociated molecules. The Answer is:
d) K2SO4 is a salt and will not react with H+ ions, so it will not affect the percent ionization of H2SO4.
Option a) SO3 can react with water to form H2SO4, which will increase the concentration of undissociated H2SO4 and decrease the percent ionization.Option b) Na2SO3 and option c) CaSO3 can both react with H+ ions to form H2SO3, which is a weaker acid and will decrease the concentration of H+ ions available for ionization of H2SO4, leading to a decrease in percent ionization.About molecules
Molecule is the smallest part of a compound that is composed of a combination of two or more atoms. Molecules are divided into two, namely compound molecules and elemental molecules. The difference between compound molecules and elemental molecules is the elements that compose them.
You can learn more about Molecules at https://brainly.com/question/29013538
#SPJ11
Where can one find most of the world's drinking water? Question 4 options: freshwater collection areas. rain clouds , manmade reservoirs , oceans
Answers
Fresh water collection areas is the area where you can find most of the
world's drinking water.
What is Freshwater?
This is the type of water which contains low amount of dissolved salts and
solid particles which makes it very suitable for drinking.
Examples of Freshwater collection areas include the following:
RiversLakes StreamsGroundwaterRead more about Freshwater here https://brainly.com/question/20458567
Nuclear fission occurs when _______________ a. TNT and plutonium are combined, causing the molecules to separate. b. a nucleus breaks up into two equal fragments that release and separate more atoms. c. like atoms collide to create double nuclei. d. trinitite is created by multiple molecules that form a single atom.
Answers
Nuclear fission occurs when a nucleus breaks up into two equal fragments that release and separate more atoms. So, the correct option is B.
Nuclear fission is a process in which the nucleus of an atom breaks apart into two or more smaller nuclei. This process releases a significant amount of energy.
Option B accurately describes the process of nuclear fission. When a heavy nucleus, such as uranium-235 or plutonium-239, absorbs a neutron, it becomes unstable and splits into two smaller nuclei.These smaller nuclei, along with additional neutrons, are released in the process. The release of neutrons can trigger a chain reaction, where each neutron released can potentially collide with other nuclei, causing them to undergo fission as well.The energy released during nuclear fission is due to the conversion of a small amount of mass into a large amount of energy, as described by Einstein's famous equation, E=mc².This energy is utilized in various applications, including nuclear power generation and nuclear weapons. Nuclear fission reactions are carefully controlled in nuclear power plants to ensure the sustained release of energy without leading to uncontrolled chain reactions. Hence the correct option is B.
For more questions on nucleus
https://brainly.com/question/29855834
#SPJ8
which of the following statements is not true?
A. covalent compounds have low melting and boiling points.
B. covalent bonds occur between nonmetals.
C. covalent compounds are often gases or liquids.
D. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules.
Answers
Answer:
I think option (D)is not true
HELP HELP HELPP! Based on the formula of kinetic energy, how will the temperature change if you increase the average velocity of the molecules in a gas?

Answers
Answer:
Explanation:
In a hot gas, the molecules move faster than in a cold gas; the mass remains the same, but the kinetic energy, and hence the temperature, is greater because of the increased velocity of the molecules. ... We can sense that one gas is hotter than another gas and therefore has a higher temperature.
The specific heat capacity of solid copper metal is 0. 385 J/g-K. How many joules of heat are needed to raise the temperature of a 2. 12-kg block of copper from 25. 0 °C to 88. 0 °C?
Answers
Answer:
Explanation:
To calculate the amount of heat needed to raise the temperature of the copper block, we can use the formula:
Q = m * c * ΔT
where Q is the amount of heat in joules, m is the mass of the copper block in grams, c is the specific heat capacity of copper in J/g-K, and ΔT is the change in temperature in Kelvin.
First, we need to convert the mass of the copper block from kilograms to grams:
m = 2.12 kg * 1000 g/kg = 2120 g
Next, we need to calculate the change in temperature in Kelvin:
ΔT = (88.0 °C + 273.15 K) - (25.0 °C + 273.15 K) = 336.30 K - 298.15 K = 38.15 K
Now, we can plug in the values into the formula:
Q = m * c * ΔT = 2120 g * 0.385 J/g-K * 38.15 K = 31233.98 J
Therefore, approximately 31,234 joules of heat are needed to raise the temperature of the 2.12-kg block of copper from 25.0 °C to 88.0 °C.
A piece of iron is heated to 120'C then placed in a calorimeter containing 50g of water. The water temperature is raised from 30°C to 40°C. Assuming the specific heat of water is 4. 18 J/8'C and the specific heat of
Iron is 0. 44 J/g C, what is the mass of the piece of iron?
118. 8
59. 45
39. 6
752
Answers
Answer:
Mass of iron = 59.375 gm
Explanation:
Calories ( or joules) are added to the water by the hot steel so at the endpoint they are BOTH at 40 C
The water gains:
4.18 j/g-C * 50 * (40-30 C) = 2090 j
The steel gave up 2090 j going from 120 to 40 C
2090 = .44 j/g-C * m * (120-40) solve fro m = 59.375 gm
A construction worker uses a pulley and a rope to
raise an 85 kg person to a height of 2 m in 6 s. How
much did he do?
Answers
Answer: Work is 1.7 kJ, Power is 280 W
Explanation: Work W = mgh, power P= W/t
W = 85 kg·9.81m/s²·2 m = 1667.7 J
P = 1667.7 J / 6 s = 277.95 W
Why is carbon dioxide called a gas and not vapour?
Answers
Explanation:
CO2 is called as gas because it exist in single thermodynamics state i.e CO exist in gases state only at room temperature.
true/false. the center of gravity of the 50-lb sign is at point g. determine the magnitude of the tension in wire de (in units of lb) necessary to hold the sign in equilibrium. the collar at a is smooth.
Answers
Answer:
False
Explanation:
in general, how do the periodic properties of the d-block elements compare with those of the main - group elements?
Answers
The periodic properties of the d-block elements differ from the main group elements in that they are less sensitive and less reactive.
The periodic table is divided into blocks; s-block, p-block, f-block, and d-block. The d-block elements are also known as transition metals.
The s and p-block elements are known as the main group elements. Compared to these, the d-block elements have some different properties because of their partially filled d-orbitals.
However, the d-block elements still have many similar properties. These elements can still displace hydrogen from dilute acid and some of them can react with water under appropriate conditions.
The first row of these transition metals are found to be more reactive than the second and third row. However, they are not as reactive as the s-block and p-block elements.
To learn more about d-block elements; click here:
https://brainly.com/question/12346980
#SPJ4