true or false: in major keys, the tonic, subdominant, and dominant triads are major.
Answers
True. In major keys, the tonic (I), subdominant (IV), and dominant (V) triads are major.
This is because the major scale follows a specific pattern of whole and half steps, and the tonic, subdominant, and dominant notes fall on the first, fourth, and fifth scale degrees respectively.
When these notes are harmonized to form triads, they naturally result in major chords. However, it's important to note that in minor keys, the subdominant and dominant triads are often different (minor or diminished) than in major keys.
True, in major keys, the tonic, subdominant, and dominant triads are major. In a major key:
1. Tonic triad (I) is formed using the 1st, 3rd, and 5th scale degrees and is major.
2. Subdominant triad (IV) is formed using the 4th, 6th, and 1st scale degrees and is also major.
3. Dominant triad (V) is formed using the 5th, 7th, and 2nd scale degrees and is major as well.
These three triads are crucial in establishing the tonality of a major key and are all major in quality.
For more information on tonality visit:
brainly.com/question/10410442
#SPJ11
Related Questions
1. You may be using medium for shoot regeneration from leaf explants of a plant in Expt-5. The plant media may contain the plant growth regulators (hoones) BA and NAA. The molecular weight of BK is 72 A : and NAA is 186. The media is pH to 5.8. (a) Before making the plant media, you found the pH to be 3.6. What would you add quiekly to get it to a pH of 5.8 (give a specific name of the solution)? Why? (1 pt) (b) How much BA will be weighed fot a 1M solution? (Y po) (c) Convert your answer from (b) to mg/ml. (Y/ pt) (d) Convert your answer from (c) to mg 1 . (1 pt) (e) How much BA will be weighed for a 5mM solution? (1/4pt) (f) Convert your answer from (c) to mg/ml. ( /4pt ) (g) Convert your answer from (f) to mg/L. (H/ pt) (h) Your stock solution of BA is 5mM and your working solution is 0.2mg/.. What volume of the stoc be added to 250ml of medium? [Hint: fook at the previous answers Keep to 4 decimal pts.) (3 pts Convert your answer from (h) to μI, and which pipettor will you use to aliquot the B. A? (1 pt)
Answers
(a) To get the pH of the media to 5.8, you would add NaOH solution. NaOH is used as a basic solution, and when it is added to a solution, it will increase the pH of the solution.
(b) The molecular weight of BA is 225.3. To prepare a 1M solution, you would have to weigh out 225.3 grams of BA.(c) To convert a 1M solution of BA to mg/mL, you can use the following equation: 1 mole = molecular weight in grams; 1000 millimoles = 1 mole. So, 1 M = 1000 mg/mL. Therefore, a 1M solution of BA is equivalent to 1000 mg/mL .(d) To convert a concentration of 1000 mg/mL .
Therefore, to calculate the weight required for a 5 mM solution, use the following formula :Mass of BA = molarity × volume × molecular weight= 5 × 0.001 × 225.3= 1.1265 grams(f) To convert a concentration of 5 mM to mg/mL, we use the following formula: Concentration (mg/mL) = (Concentration (mM) × Molecular weight) / 1000= (5 × 225.3) / 1000= 1.1265 mg/mL(g)
To convert a concentration of 1.1265 mg/mL to mg/L, we multiply by 1000, so 1.1265 mg/mL = 1126.5 mg/L.(h) Given that the stock solution of BA is 5 mM and the working solution is 0.2 mg/mL.
To know more about increase visit:
brainly.com/question/19383315
#SPJ11
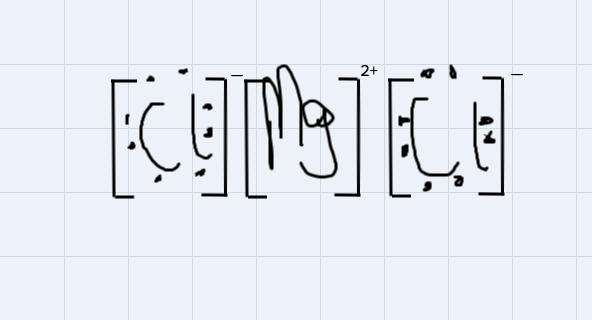
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
Swift has a 1.43mol sample of carbon dioxide. If the pressure of the sample is 34.56 kPa, and the volume is 440.99 mL, what will the temperature of the sample be in Kelvin?
Answers
For this problem, we could use the ideal gas equation:
\(PV=nRT\)Where P is the pressure, V is the volume, R is a constant and T is the temperature.
Before we start solving, we should remember the units:
P [atm]
V [L]
What is the si unit if sound
Answers
Explanation:
The SI Unit of sound is decibel.
or we can say that
The SI Unit of sound is watt per square metre (i.e.w/m2).
What is said to have happened to the electrons in an atom in its ground state absorbs a quantum of energy from light
Answers
Answer:
The electron from the ground state to occupy a next energy level. In this case,we say that the electron is excited
The atomic number of an element is equal to the number of electrons found in an atom of that element.
True or false
Answers
Answer:
False.
Explanation:
The atomic number is equal to the number of Protons found in an atom.
Answer:
TrueExplanation:
If the element that you have has a neutral atom then- Atomic number is equal to the protons
- Protons are equal to electrons.
So, the answer would be TRUEHope this helped,
Kavitha Banarjee
name two substances that undergo melting
Answers
Answer:
they ate lelo pudina hahahha
PLEASE HURRY !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! WILL GIVE BRAINLIEST
What type of rock is limestone? Describe how a limestone rock is likely to change over a long period of time.
Answers
Answer:
Over time, the limestone is broken down into its chemical parts and may come back to the surface as volcanic CO2 (in the atmosphere). From the atmosphere, the CO2 might again become part of the biosphere by molluscs or corals absorbing the CO2 to help make their shells
Explanation:
Over a long period of time, we would take a look at the rock "limestone" through the rock cycle. Limestone being a sedimentary rock would be converted to marble, a metamorphic rock if subjected to metamorphic conditions over an extensive period of time.
mercury thermometer should not be placed in the mouth of the children why?
Answers
Answer: If a pinch of mercury is consumed you will die from it so using a mercury thermometer is very unsafe if its breaks and a child consumes that mercury.
Explanation:
Don't use one
If the glass breaks and the mercury is not properly cleaned up, the little silvery ball within a mercury thermometer might be hazardous. As the mercury evaporates, it may pollute the air around and turn dangerous to both people and animals.
Children that have been exposed to mercury have lower IQs, hearing impairments, and worse coordination.
Long-term exposure worsens and exacerbates symptoms, which may lead to personality changes or even coma.
Mercury thermometers can be replaced by a number of things:
electronic thermometersGlass thermometers with gallium tinalcohol thermometers in glassThese non-mercury fever thermometers are significantly safer and equally accurate as mercury thermometers.
Read more about Mercury Thermometers :
https://brainly.com/question/27323100
How do the physical and chemical properties of wood contribute to the function of the synthetic products made of wood?
Answers
Wood's tensile strength is reduced. Another risk factor for the strength of the wood is the loading time. The rigidity of wood is affected by temperature.
What is wood?Wood is one of the most common and useful natural materials. It is the main tissue that supports and conducts nutrients in trees and other plants. Wood comes in a variety of colours & grain patterns and is produced by numerous botanical species, comprising both gymnosperms & angiosperms.
Its wood's tensile strength is reduced. Another risk factor for the strength of the wood is the loading time. The rigidity of wood is affected by temperature. Unfavourable weather and unexpected temperatures will have an impact on the wood's strength. Among its attributes are mechanical, chemical, and physical qualities.
Therefore, wood's tensile strength is reduced. Another risk factor for the strength of the wood is the loading time.
To know more about wood, here:
https://brainly.com/question/10967023
#SPJ2
12. The molar mass of water is 18.0
g/mol. A mass of 90.0 grams of
water is equivalent to how many
moles?
HELP ASAP!!!

Answers
Answer:
5moles
Explanation:
number of moles=mass/molar mass
The molar mass of water is 18.0 g/mol. A mass of 90.0 grams of water is equivalent to 5 moles. Therefore, option B is correct.
What is molar mass ?The mass in grams of one mole of a substance is defined as its molar mass. Molar mass is measured in grams per mole, abbreviated as g/mol. The isotopic atomic mass of a single isotope of any given element is a value that compares the mass of that isotope to the mass of the isotope carbon-12.
A compound's molar mass can be calculated by adding the standard atomic masses (in g/mol) of its constituent atoms.
A compound's molar mass indicates the mass of one mole of that substance. In other words, it tells you how many grams of a compound there are per mole. As a result, the units for molar mass are grams/mole.
number of moles = mass / molar mas
= 90.0 grams / 18.0grams
= 5 moles
Thus, option B is correct.
To learn more about the molar mass, follow the link;
https://brainly.com/question/12127540
#SPJ6
Arrange these places in order of how safe they are to live in (safest first): Japan, Spain, Ireland, Australia, Iceland.
Answers
According to World Population Review 2022 the safest country to live are as follows.
Iceland is the safest amongst all.IrelandJapanSpainAustralia is least safe in this particular list.These countries are listed safe by calculating global peace index.
What is global peace index?This index measures the nation's and regions peacefulness and is finally reviewed by Institute of Economic and Peace (IEP).
The role of IEP is to measure peace at the worldwide and country level degree and lets us to evaluate the social, political and financial elements that create peace. Each year the Institute for Economics and Peace produces the Global Peace Index, the world’s main degree of countrywide peacefulness, rating 163 nations in line with their ranges of peace.
To know more about global peace click on:
brainly.com/question/16689991
#SPJ9
An atom typically exists in an excited state for about Δ t = 10 − 8 s. What is the minimum uncertainty in the energy of the state (in eV)?
Answers
The minimum uncertainty in the energy of the excited state is approximately 0.206 eV.
According to the Heisenberg uncertainty principle, there is an inherent uncertainty in the measurement of certain pairs of physical properties, such as the position and momentum or the energy and time of a quantum system.
The uncertainty principle can be expressed mathematically as ΔE Δt ≥ ħ/2, where ΔE is the uncertainty in the energy of the system, Δt is the uncertainty in the time interval over which the energy is measured, and ħ is the reduced Planck constant.
In this case, the given time interval is Δt = 10^(-8) s. We can use this value to calculate the minimum uncertainty in the energy of the excited state:
ΔE Δt ≥ ħ/2
ΔE ≥ ħ/(2Δt)
ΔE ≥ (6.626 x 10⁻³⁴ J s)/(2 x 10⁻⁸ s)
ΔE ≥ 3.313 x 10⁻²⁶ J
To convert this energy uncertainty to eV, we can use the conversion factor 1 eV = 1.602 x 10⁻¹⁹ J:
ΔE = (3.313 x 10⁻²⁶ J) / (1.602 x 10⁻¹⁹ J/eV)
ΔE ≈ 0.206 eV
Therefore, the minimum uncertainty in the energy of the excited state is approximately 0.206 eV. This means that the energy of the excited state can fluctuate by up to this amount over the time interval Δt = 10⁻⁸ s.
To know more about Heisenberg uncertainty principle, refer to the link below:
https://brainly.com/question/30402752#
#SPJ11
Which of the following is the net ionic equation for the reaction between aqueous sodium fluoride and hydrochloric acid? (A) NaF (aq) + HCI (aq) → Naci (aq) + HF (aq) (B) Na+ (aq) + F- (aq) + H+ (aq) + CI+ (aq) → Na+ (aq) + Cl - (aq) + HF (aq) (C) Na+ (aq) + CI+ (aq) → NaCl (aq) (D) F- (aq) + H+ (aq) → HF (aq)
Answers
The net ionic equation for the reaction between aqueous sodium fluoride and hydrochloric acid is B. Na+ (aq) + F- (aq) + H+ (aq) + Cl- (aq) → Na+ (aq) + Cl- (aq) + HF (aq)
This is the net ionic equation for the reaction between aqueous sodium fluoride and hydrochloric acid. The balanced equation for this reaction is NaF (aq) + HCl (aq) → NaCl (aq) +HF (aq).
The net ionic equation is derived by canceling out the spectator ions, which are ions that don't participate in the chemical reaction. In this case, Na+ and Cl- are spectator ions, which means that they don't participate in the reaction and are present in the same form on both sides of the equation. Therefore, they are cancelled out, leaving the net ionic equation Na+ (aq) + F- (aq) + H+ (aq) + Cl- (aq) → Na+ (aq) + Cl- (aq) + HF (aq).
Learn more about net ionic equations here: https://brainly.com/question/19705645
#SPJ4
Which question can be asked to determine if a wave is electromagnetic or mechanical
Answers
Answer: The Answer is
Does it require medium to travel
Explanation:
Answer:
does it require a medium
Explanation:
trust me
middle one! please help. i’ll do anything.

Answers
Answer:
I believe the answer would be weathering
Answer:
Erosion
Explanation:
Because....
Deposition is the action of deposing someone, especially a monarch.
weathering is the process of wearing or being worn by long exposure to the atmosphere.
Why is blood liquid?
Answers
Answer:
ok here is you answer
Explanation:
Blood is a liquid because it is composed of cells and plasma that are suspended in a liquid state and can easily flow through the circulatory system, delivering oxygen and nutrients to cells and removing waste products.
mark me as brainliestA trench may form at a transform fault.
O True
O False
Answers
Answer:
True
Explanation:
Because yes
!!!!!!
Describe the difference between naturally occurring radiation and man-made
radiation.
Answers
Answer:
Man-made radiation is generated in range of medical, commercial and industrial activities and natural occuring radiation occurs due to minerals.
A group of students working in a chemistry lab are planning a procedure to
neutralize hydrochloric acid (HCI, strong acid). How should they BEST accomplish
this?
Answers
use a strong base to neutralize, like NaOH, KOH, etc
____a region in an atom where there is a high probability of finding electrons
Answers
Atomic orbitals are places on an atom where there is a very high chance of finding electrons. Electrons are located in the area termed the electron cloud that surrounds the nucleus of an atom.
The set of primary particles that comprise an atom includes many other particles than the electron. Like other elementary particles, electrons can collide with other particles and, when they are diffracted, exhibit wavelike characteristics. Atomic orbitals are places on an atom where there is a very high chance of finding electrons. A chemical element is made up of an atom, which is the smallest building block of ordinary matter. Every substance—solid, liquid, gas, and plasma—is made up of neutral or ionised atoms. The average atomic diameter is 100 picometers or less.
Learn more about electrons here
https://brainly.com/question/29757010
#SPJ4
If 613.28 mL of 2.744 M of aluminum hydroxide reacts with 10.35 g of ammonium persulfate in a chemical reaction. Find the pressure of the gas produced if you managed to collect 1536.70 mL of it at 42.455 °C. Show 2 decimal places.
Answers
The pressure of the gas produced is approximately 587.17 kPa.
How to find the pressure of the gasTo solve this problem, we first need to find the amount of gas produced by the reaction of aluminum hydroxide with ammonium persulfate, then use the Ideal Gas Law (PV = nRT) to calculate the pressure.
First, let's find the number of moles of ammonium persulfate by using the molar mass:
10.35 g ÷ (2 * (1 + 32 + 64 + 16)) g/mol = 0.108 mol
Next, let's find the number of moles of aluminum hydroxide:
613.28 mL * 2.744 M = 1692.04 mol
Now, let's assume that the reaction goes to completion and that all the aluminum hydroxide reacts with ammonium persulfate, so the number of moles of gas produced will be equal to the number of moles of ammonium persulfate:
0.108 mol
Finally, we can use the Ideal Gas Law to calculate the pressure:
P = (n * R * T) / V
where n = 0.108 mol, R = 8.31 J/mol K, T = (42.455 + 273.15) K, and V = 1536.70 mL * 10^-3 L
P = (0.108 * 8.31 * (42.455 + 273.15)) / (1536.70 * 10^-3)
P = (0.108 * 8.31 * 315.605) / (1.5367)
P = 905.752 / 1.5367
P = 587.17 kPa
So, the pressure of the gas produced is approximately 587.17 kPa.
Read more about pressure here:
https://brainly.com/question/28012687
#SPJ1
#7) How many waves are in this picture?

Answers
Answer:
4
Explanation:
Answer:
B.
Explanation:
I don't know much about this subject, but it seems that a wave is when the line is above the line in the middle.
Please help with B, I don't know how to find the molar mass.

Answers
Answer:
The molar mass of NO (nitrogen monoxide) is approximately 30.01 g/mol.
Explanation:
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). The molar mass of NO (nitrogen monoxide) can be found by adding up the atomic masses of nitrogen (N) and oxygen (O), which can be found on the periodic table of elements. The molar mass of NO is approximately 30.01 g/mol.
HURRY !!!! Technology can often be used to extend and enhance human abilities. Sometimes, different types of technology can even be used for a similar purpose. For example, length can be measured using a metric ruler, a meter stick, a tape measure, or a micrometer. Which of these instruments would most precisely measure extremely small objects? A-metric ruler B-meter stick C-tape measure D-micrometer
Answers
answer:
c. tape measure
because a tape measure can extend and retract and can measure in inches, half inches, centimeters and more small measurements!
explanation:
hope this helped <3 also if wouldn't mind could you pls give me brainliest? (im trying to level up) thanks! :)
Answer:
D
Explanation:
Tape measures and meter sticks are usually used to measure long distances. A metric ruler is usually used to measure lengths that are less than 30 cm. A micrometer is used to precisely measure small objects.
To use a micrometer, an object is inserted between two rods. Then, the moveable rod is twisted, like a screw, until it is positioned snugly against the object. Finally, the object's length is measured using the scale on the micrometer.
so i hope this helps
1. A student in lab measures 4.6 grams of copper for an experiment. Upon further analysis he determines that he should have measured out 4.7 grams of copper. What is his percent error?
Answers
Answer:
\(2.13\%\)
Explanation:
Quantity of copper measured by a student = 4.6 grams
Original quantity of copper = 4.7 grams
Error in measurement = Original quantity of copper - Quantity of copper measured by a student \(=4.7-4.6=0.1\) grams
To find the percent error, apply the following formula:
Percent error = (Error in measurement / Original quantity of copper) × 100
\(=\frac{0.1}{4.7}(100)=2.13\%\)
Of the following, indicate which are pentose sugars.
- D-Threose Glyceraldehyde
- D-Fructose
- L-Glucose
- D-Sorbose
- None of the Above
Answers
Of the given options, pentose sugar is D-Sorbose.
Pentose sugars: These are a class of sugars that have five carbon atoms in their molecular structure. Pentose sugars are one of the two most common types of sugars found in living organisms, the other being hexose sugars. DNA and RNA, as well as many coenzymes, contain pentose sugars.
The pentose sugars are-Ribose, Deoxyribose, Xylose, Arabinose, etc. Glyceraldehyde is a triose sugar, and fructose and glucose are hexose sugars. L-glucose, on the other hand, is an unnatural isomer of glucose that has no known biological function. The correct answer is: D-Sorbose.
"pentose sugars", https://brainly.com/question/30453184
#SPJ11
in a food web, energy and matter are passed on to the next trophic level when an
organism feeds on another organism. However, none of the organisms consume the top
consumers.
How does the top consumer help in returning matter to the environment?
O by releasing body heat in various metabolic processes
O by excretion of waste and the death of the top consumer
O by utilizing atmospheric oxygen to form energy molecules
O by competition with other top consumers for food
Answers
Answer:
O by excretion of waste and the death of the top consumer
Explanation:
The top consumers in the food web pass energy and matter back to the next trophic level when they excrete waste products and also when they die.
Excretion is the passing out of waste materials of metabolism.
During the death of an organism, organic materials are liberated back into the ecosystem. Carbon is released into the soil, carbon dioxide into the atmosphere etc.
These materials are then recycled back into the ecosystem by the activities of plant and special organisms called decomposers. These organisms feed on dead animals especially.
1.35 soda preference: you would like to conduct an experiment in class to see if your classmates prefer the taste of regular coke or diet coke. briefly outline a design for this study.
Answers
To determine the statistical analysis is a difference between the groups we have to Calculate the number of participants who preferred each soda
Experiment design for studying soda preference A well-designed experiment typically involves identifying a problem, designing a study that will yield data to answer the research question, and collecting and analyzing data.
In this case, you would like to conduct an experiment in class to see if your classmates prefer the taste of regular coke or diet coke. The following is an experiment design for this study.
Step 1: Develop a research question and hypothesis. The research question in this study is “Which soda do my classmates prefer, regular coke or diet coke?”The hypothesis of this study is that more students will prefer regular coke to diet coke.
Step 2: Select a sample of participants. A sample of participants should be chosen for the study. The sample should be large enough to provide sufficient data but small enough to be manageable. In this case, you could select a sample of 50 participants.
Step 3: Divide participants into two groups. Divide the participants randomly into two groups, with each group containing an equal number of participants. One group will be given regular coke, while the other group will be given diet coke.
Step 4: Ask participants to taste their assigned soda. Once the participants are divided into groups, give each participant a cup of the soda they have been assigned to taste. Be sure that each participant does not know which soda they are tasting to avoid any bias.
Step 5: Collect data. After the participants have tasted their assigned soda, ask them which one they preferred. Record their answers and tally the results.
Step 6: Analyze the data. Calculate the number of participants who preferred each soda. Use statistical analysis to determine whether there is a statistically significant difference between the groups.
To know more about statistical analysis refer here:
https://brainly.com/question/32467087#
#SPJ11
2.8g of silicon react with 3.2g of oxygen to give a compound, which is shown below. The relative atomic mass of silicon is 28 and of oxygen is 16.What is the value of y in the formula below? SIOy
Answers
The value of Y in the formula when 2.8g of silicon react with 3.2g of oxygen, is 2
The numbers in the formula of a compound determine the ratio of moles of each element in the compound.
Given:
2.8g of silicon
3.2g of oxygen
[relative atomic mass of silicon is 28 and of oxygen is 16]
For silicon, n = 2.8 ÷ 28 = 0.1 (n= moles)
For oxygen, n = 3.2 ÷ 016 = 0.2
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.1 moles.
For Silicon = 0.1/0.1 = 1
For Oxygen = 0.2/0.1 = 2
Taking the mole ratio as their subscripts.
The ratio of Si:O is 1:2
SiO\(_{2}\) is the formula of silicon dioxide.
The value of y in the formula SiO\(_{Y}\) is 2 when 2.8g of silicon react with 3.2g of oxygen.
To know more about moles, click on this link
https://brainly.com/question/22540912