what are the spectator ions in the reaction between mg(oh)2 (aq) and hcl (aq)? group of answer choices mg2 and cl- h and oh- oh- only h and cl- mg2 and h
Answers
The spectator ions in the REACTION between Mg(OH)2(aq) and HCl(aq) are OH-.
Here is the balanced chemical equation for the reaction:
Mg(OH)2(aq) + 2HCl(aq) → MgCl2(aq) + 2H2O(l)
In this reaction, Mg(OH)2(aq) reacts with HCl(aq) to produce MgCl2(aq) and H2O(l). Mg2+ and Cl- are involved in the reaction, but they are not spectator ions because they are part of the products. OH-, on the other hand, does not participate in the reaction and is present on both sides of the equation, making it a spectator ion.
Thus, the spectator ions in the reaction between Mg(OH)2(aq) and HCl(aq) are OH-.
To know more about chemical visit :-
https://brainly.com/question/29886197
#SPJ11
Related Questions
Describe the physical characteristics of water that make it a valuable resource for agricultural, industrial, and domestic uses.
Answers
Water is a vital component of agricultural productivity and is crucial to the security of our food supply. 20% of the total is made up of irrigated agriculture.
What role does water have in agriculture and humans?We can grow fruits, vegetables, and cattle, which makes up a significant portion of our diet, thanks to the usage of agricultural water. Agricultural water is used for irrigation, the application of pesticides and fertilizers, cooling the crop (for instance, light irrigation), and frost management.
What residential uses does water have?Drinking, cooking, washing hands and bodies, washing clothes, cleaning cooking utensils, cleaning the house, watering animals, irrigating gardens, and frequently for business activities are just a few of the various uses of water in households.
To know more about physical characteristics of water visit :-
https://brainly.com/question/28646615
#SPJ4
When the first American astronauts were planning to walk on the Moon, they knew that the gravity on the Moon was less than the gravity on Earth. With this information, what did the astronauts expect to be MOST different on the Moon? Select one: a. their mass b. their height c. their weight d. their volume
Answers
Answer:
Their weight.
Explanation:
Weight is the measurement of gravity pushing you down, so with less gravity there would be less weight.
The astronauts expect their weight to be different on the Moon
Definition of weightWeight is simply defined as the gravitational pull of the earth on an object. It is measured in Newton.
Description of weightThe weight of an object varies with location because of gravity.
The weight of an object is related to it's mass according to the following equation:
Weight = mass × Acceleration due to gravity
Since the gravity of the Moon is lesser than that of the Eearth, the astronauts certainly knew that their weight will be different on the Moon.
Learn more about weight:
https://brainly.com/question/1839641
What is the major product: cis or trans? why? (reduction lab)
Answers
Trans isomer is generally the major product due to its higher stability compared to cis isomer.
What determines the major product in a reaction forming cis and trans isomers?
The major product formed in a reaction depends on several factors such as the reactants' electronic and steric effects, the reaction conditions, and the mechanism involved. In the case of a reaction that forms cis and trans isomers, the major product will be the one that is more stable. This stability depends on the relative positions of the substituents and their interactions with each other.
Generally, trans isomers are more stable than cis isomers due to the absence of steric hindrance between the substituents. The bulky substituents in cis isomers can cause repulsion and destabilize the molecule. Therefore, the major product in this case would typically be the trans isomer. However, there are exceptions where the cis isomer may be the major product due to specific reaction conditions or steric effects that stabilize the cis isomer.
To learn more about Trans isomer, visit: https://brainly.com/question/30640902
#SPJ4
a two-year chart of carbon dioxide measurements made from the top of mauna loa, hawaii, shows a saw tooth pattern. why?
Answers
The sawtooth pattern observed in the two-year chart of carbon dioxide measurements from Mauna Loa, Hawaii, is a result of seasonal variations and human activities.
Carbon dioxide levels increase during the winter months when plants are dormant and decrease during the summer months when they are actively photosynthesizing. Additionally, human activities such as burning fossil fuels and deforestation contribute to the overall increase in carbon dioxide levels. The sawtooth pattern provides valuable data for scientists studying the impacts of climate change and global warming. It also serves as a reminder of the urgent need to reduce carbon emissions and adopt sustainable practices to mitigate the effects of climate change.
The sawtooth pattern observed in the two-year chart of carbon dioxide (CO2) measurements from Mauna Loa, Hawaii, is primarily due to seasonal fluctuations in plant growth and decay. During spring and summer, increased photosynthesis in the Northern Hemisphere absorbs CO2 from the atmosphere, causing a decrease in CO2 levels. Conversely, during fall and winter, reduced photosynthesis and increased plant decay release CO2 back into the atmosphere, resulting in a rise in CO2 levels. This cyclical pattern creates the sawtooth appearance on the chart, while the overall trend still shows a continuous increase in atmospheric CO2 levels due to human activities.
To know about sawtooth:
https://brainly.com/question/31595132
#SPJ11
an acid such as hydrochloric acid (hcl) that ionizes freely, gives up most of its hydrogen ions, and can markedly lower the ph of a solution is known as what type of acid?
Answers
An acid such as hydrochloric acid (HCl) that ionizes freely and gives up most of its hydrogen ions is known as a strong acid. Strong acids have a very low pH and can markedly lower the pH of a solution, making it more acidic.
When dissolved in water, HCl dissociates almost completely into H+ and Cl- ions, making it a strong electrolyte. Other examples of strong acids include sulfuric acid (H2SO4), nitric acid (HNO3), and hydroiodic acid (HI). Strong acids are important in many chemical reactions and are commonly used in laboratories and industries. However, they can also be hazardous and must be handled with care. The strength of an acid is related to its ability to donate hydrogen ions (protons), and is measured on a scale called the pH scale. The pH scale ranges from 0 to 14, with 7 being neutral. Acids have a pH lower than 7, while bases have a pH higher than 7.
learn more about acids
https://brainly.com/question/17461457
#SPJ11
What is the mass of 2.13 moles of lithium arsenate
Answers
Introductory physics
An element with an atomic number of 88 goes through alpha decay.
What is it's atomic number now?
Answers
The answer would be 86
When a student dissolves 2.50 g of LiCl in 100.0 mL of water (100.0 g) the temperature rises from 24.0 oC to 29.11oC. What is ∆H in KJ/mol for the dissolution of LiCl in water? Make sure you include the correct sign for ∆H and units (with a space between number and unit)
Answers
Answer:
\(36.273\ \text{kJ/mol}\)
Explanation:
m = Mass of LiCl = 2.5 g
M = Molar mass of LiCl = 42.394 g/mol
c = Specific heat of water = \(4.186\ \text{J/g}^{\circ}\text{C}\)
\(\Delta T\) = Change in temperature = \(29.11-24=5.11\ ^{\circ}\text{C}\)
\(m_w\) = Mass of water = \(\rho V=1\times 100=100\ \text{g}\)
Number of moles
\(n=\dfrac{m}{M}\\\Rightarrow n=\dfrac{2.5}{42.394}\\\Rightarrow n=0.05897\ \text{mol}\)
Heat is given by
\(Q=m_wc\Delta T\\\Rightarrow Q=100\times 4.186\times 5.11\\\Rightarrow Q=2139.046\ \text{J}\)
Enthalpy is given by
\(\Delta H=\dfrac{Q}{n}\\\Rightarrow \Delta H=\dfrac{2139.046}{0.05897}\\\Rightarrow \Delta H=36273.46\ \text{J/mol}=36.273\ \text{kJ/mol}\)
The enthalpy for the dissolution is \(36.273\ \text{kJ/mol}\).
which of the following is an example of a solution?
a. fog
b. soda water
c. milk
d. mud
Answers
Answer:
B
Explanation:
a solution is a mixture of more than one solvent, in this case: soda water
Answer:
b soda water
Explanation:
because what the last guy said
How many electrons are present in the nonbonding π molecular orbital of the allyl anion? a.2 b.1 c.3 d.0
Answers
There are 2 electrons present in nonbonding π molecular orbital of the allyl anion.
Frequently mentioned as reaction intermediates include allylic radicals, anions, and cations. Each one has three adjacent sp2-hybridized carbon centers, and they all rely on resonance for stability. Two resonance structures could be used to present each species, with the charged and unpaired electron scattered across both the 1,3 and 0 positions.
A total of two pi electrons are present in the allyl cation; in the resonance, there are two electrons in the pi bond and none on the allyl carbon. Keep in mind that the allyl cation's lowest-energy molecular orbital, 1, is its "highest occupied" molecular orbital.
Therefore, there are 2 electrons present in nonbonding π molecular orbital of the allyl anion.
To know more about molecular orbital
https://brainly.com/question/28166892
#SPJ4
Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all specles within the reaction. As(s)→AsH ( s)
Answers
The complete and balanced half reaction in acidic solution is :
As(s) + 3H2O(l) → AsH3(s) + 3OH^-(aq)
To complete and balance the half-reaction: As(s) → AsH3(s) in acidic solution, we need to account for the gain of hydrogen (H) on the arsenic (As) atom. Here's the balanced half-reaction:
As(s) + 3H2O(l) → AsH3(s) + 3OH^-(aq)
To balance the half-reaction, we need to ensure that the number of atoms and charges are balanced on both sides. Here's the step-by-step process:
Step 1: Balance the arsenic (As) atoms:
As(s) → AsH3(s)
Step 2: Balance the hydrogen (H) atoms:
As(s) + 3H2O(l) → AsH3(s)
Step 3: Balance the oxygen (O) atoms by adding water (H2O):
As(s) + 3H2O(l) → AsH3(s) + 3H2O(l)
Step 4: Balance the charges by adding hydroxide ions (OH^-):
As(s) + 3H2O(l) → AsH3(s) + 3OH^-(aq)
Now, the half-reaction is balanced. Note that the phases are also included: (s) for solid, (l) for liquid, and (aq) for aqueous.
Learn more about half-reaction https://brainly.com/question/26411933
#SPJ11
What are the 2 products of combustion always?
Answers
helpp!!
What pair of elements is most likely to form a metallic bond?
a.sodium and arsenic
b.lithium and oxygen
c.magnesium and zinc
d. calcium and selenium
who ever answers gets the branliest !
Answers
Answer:
c.magnesium and zinc
Explanation:
you're welcome
Which of the following best explains how longitudinal and transverse waves transfer energy?
A. In both longitudinal and transverse waves the
energy is transferred horizontally.
B. In both longitudinal and transverse waves the
energy is transferred in an up and down
direction.
C. In longitudinal waves the energy is transferred in
an up and down direction, and in transverse
waves the energy is transferred horizontally.
D. In longitudinal waves the energy is transferred
horizontally, and in transverse waves the energy
is transferred in an up and down direction.
Answers
what is the difference between autotrophs and heterotrophs?
Answers
Answer:
Autotrophs have the ability to synthesize their own food from the substances surrounding them. Examples of this are photosynthesis or chemical energy. Heterotrophs cannot synthesize their own food. They have to get their nutrition from other animals or plants.
In shorter terms, autotrophs are plants. Heterotrophs are humans.
Explanation:
An air plane is flying at a crusing speed of 200 miles per hour for 3 hours. How far would the plane fly?
Answers
What is true about constructive interference?A.Amplitudes in the opposite direction interfere to make larger waves.B.Amplitudes in the same direction interference to larger waves.C.Amplitudes in the same direction interference to make smaller waves.
Answers
Answer:
B. Amplitudes in the same direction interference to larger waves
Explanation:
This is because constructive interference is the addition of waves to form a larger wave. In constructive interference, waves with amplitudes moving in the same direction add to form a larger wave.
I NEED HELP!
Can you dissolve .35 moles of Potassium Permanganate (KMnO4 ) into 500 mL of water? _________ Why? / Why not? (please show work)
Answers
When the number of moles is less than 0.35 Therefore, KMnO4 will not dissolve because the number of moles is less than 0.35 mol.
What is mole ?One mole of any substance is equal to 6.023 × 10²³ units of that substance such as atoms, molecules, or ions. The number 6.023 × 10²³ is called as Avogadro's number or Avogadro's constant.
The mole concept can be used to convert between mass and number of particles.
When we know that the solubility of KMnO4 is 6.4 g/100mL
Therefore, 6.49 g → solve in 100mL
? ← solve in 500mL
The amount of KMO4 soluble in 500mL
= (500X 6.49) /100
= 32.45 g
The molar mass of the KMnO4 = 158/mol
we can get the number of moles of KMnO4 = mass of KMnO4 / molar mass of KMnO4
number of moles of KMnO4 = 32.45 / 158
= 0.2 mol
Thus, When the number of moles is less than 0.35 Therefore, KMnO4 will not dissolve because the number of moles is less than 0.35 mol.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Chloe wants to lighten the color of her dark hair, which will require the use of both haircolor and hydrogen peroxide. Hydrogen peroxide (H2O2) is an example of a(n):
compound molecule.
elemental molecule.
immiscible liquid.
miscible liquid.
Answers
Answer:
H₂O₂
Explanation:
Hydrogen peroxide is a typical example of a compound molecule. A compound is made up of one or more atoms that are combined together in a definite grouping.
The properties of a compound is different from those of the elements that combines to from them.
A molecule is a covalent compound that has discrete or separate units. The formula of such compound represents a certain discrete entity. From this problem, we know that hydrogen peroxide is a covalent compoundwork conducted near flammable gasses or explosive materials must be conducted with?
Answers
Work conducted near flammable gases or explosive materials must be conducted with appropriate safety measures and precautions to prevent the ignition of such materials. Specifically, such work should be conducted in a well-ventilated area with adequate air exchange to prevent the buildup of flammable gases.
What is Personal protective equipment?Personal protective equipment (PPE) such as flame-resistant clothing, safety glasses, and gloves should also be worn to protect workers from potential hazards. Any ignition sources, such as open flames, sparks, or electrical equipment, should be removed or adequately shielded to prevent accidental ignition.
Name some flammable gases.Flammable gases can ignite and burn quickly in the presence of a spark or flame. Some examples of flammable gases are Hydrogen (H2), Methane (CH4), Propane (C3H8), Butane (C4H10), Acetylene (C2H2), and Ethylene (C2H4).
To learn more about flammability, visit here:
https://brainly.com/question/13323225
#SPJ1
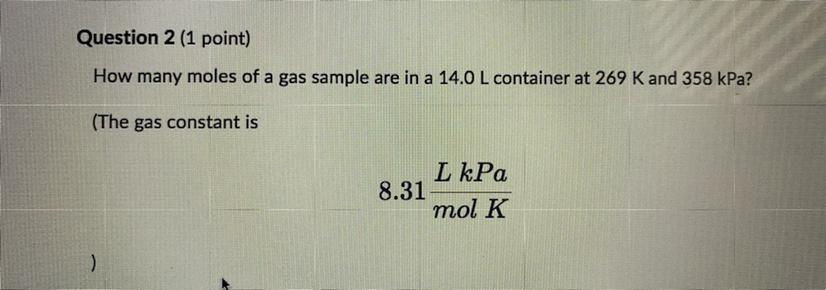
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
A separatory funnel contains ethyl acetate and an aqueous solution of some kind. What comprises the bottom layer?.
Answers
A separatory funnel contains ethyl acetate and some sort of aqueous solution. The bottom layer is made up of methylene chloride.
What is methylene chloride ?Methylene chloride, a clear, colorless, nonflammable, volatile liquid chlorinated hydrocarbon with a sweet, agreeable odor, releases highly poisonous phosgene vapours when heated to the point of disintegration.Methylene chloride is primarily used as a solvent in paint removers, but it is also used in aerosol formulations, pharmaceutical production, surface degreasing, electronic manufacturing, and ethane foam blowing.Dichloromethane can be found naturally in the ocean, macroalgae, marshes, and volcanoes. Industrial emissions, on the other hand, are the primary source of dichloromethane in the environment. The end products of these reactions are chloromethane, dichloromethane, chloroform, carbon tetrachloride, and hydrogen chloride. These chemicals are separated using distillation.To learn more about methylene chloride, refer to:
brainly.com/question/13189086
#SPJ4
What the anode , cathode and the electrolyte of a cell tha t you might use to electrolyte a spoon made from iron with silver?
Answers
The silver coating on the spoon is produced. When electrolyzing a spoon made from iron with silver, the anode, cathode, and electrolyte that can be used are as follows:
Anode: The anode is a negatively charged electrode, usually made of metal or graphite, that releases electrons during electrolysis. It is made of pure silver.Cathode: The cathode is a positively charged electrode that receives electrons during electrolysis. It is made of iron.Electrolyte: The electrolyte is a solution that conducts electricity and contains ions that can be reduced or oxidized. The electrolyte used for this process is a solution of silver nitrate (AgNO3) in water.The silver ion (Ag+) moves from the anode to the cathode through the electrolyte. At the cathode, it accepts an electron, reducing it to metallic silver (Ag). Fe(s) is oxidized to Fe2+(aq) ion at the anode, while Ag+ ions are reduced to Ag(s) at the cathode. Therefore, the silver coating on the spoon is produced.For such more questions on silver coating
https://brainly.com/question/29736740
#SPJ8
what do you think ionization means?
Answers
what is the relationship between chemical and electrical energy
Answers
Answer:
Electrochemistry,
Explanation:
Electrochemistry is a branch of chemistry concerned with the relation between electricity and chemical change. Many spontaneously occurring chemical reactions liberate electrical energy, and some of these reactions are used in batteries and fuel cells to produce electric power.
I Hope This Helps!!!!!
Answer:
Electrochemistry, branch of chemistry concerned with the relation between electricity and chemical change. Many spontaneously occurring chemical reactions liberate electrical energy, and some of these reactions are used in batteries and fuel cells to produce electric power.
how to tell if gold is real with a lighter
Answers
Answer:
Real, pure gold, when exposed to the flame, will get brighter after a while as it gets hotter, but will not darken. Fake gold pieces, such as fool's gold (actually pyrite, an iron sulfide) and pieces made of brass, iron or copper alloys will darken or otherwise change color when exposed to fire.
Explanation:
Your welcome :)
PDB CODE: 1MRY SEQUENCE POSITION: 229 AMINO ACID MUTATED TO: ARG In the PDB protein, you were given the sequence position of a particular amino acid that is mutated to another amino acid. Draw the structure of the two amino acids. Describe why this position in your protein is important and outline the effects of the mutation will have on the 3-D structure and the function of your protein.
Answers
The mutated amino acid at position 229 is arginine (Arg), and its substitution can potentially disrupt the 3D structure and alter the function of the protein due to changes in side chain properties and interactions.
Structure of the Amino Acids:
The wild-type amino acid at position 229 is not specified, so the structure cannot be provided.
The mutated amino acid is arginine (Arg), which has a side chain containing a positively charged guanidinium group.
Importance of the Position:
The specific position 229 in the protein sequence may be functionally significant, such as being involved in protein-protein interactions, binding sites, catalytic activity, or structural stability.
Without detailed knowledge of the protein, its function, and its structural context, it is difficult to determine the exact importance of this specific position.
Effects of the Mutation on Structure and Function:
The substitution of an amino acid at position 229 from the original to arginine can have various effects on protein structure and function.
Arginine's larger and positively charged side chain may introduce steric clashes or alter electrostatic interactions within the protein structure.
The mutation can potentially disrupt local or global protein folding, stability, or conformational changes, affecting its overall 3D structure.
The functional consequences of the mutation depend on the specific role of the amino acid at position 229, which can include changes in protein-protein interactions, enzymatic activity, substrate binding, or signal transduction pathways.
It is crucial to analyze the protein's structural context, available experimental data, and computational modeling techniques to gain a more accurate understanding of the specific effects of the mutation on the protein's structure and function in the given context.
Learn more about Amino Acids from the given link:
https://brainly.com/question/31872499
#SPJ11
a liquid solvent is added to a flask containing an insoluble solid. the total volume of the solid and liquid together is 91.0 ml. 91.0 ml. the liquid solvent has a mass of 21.0 g 21.0 g and a density of 0.865 g/ml. 0.865 g/ml. determine the mass of the solid given its density is 1.75 g/ml.
Answers
The mass of the solid of density 1.75 g/ mole is 116.77g. This is calculated using the expression for density.
Density is defined as the mass per unit of volume of the substance. The symbol used for density is ρ. Basically density is defined as mass divided by volume.
Density = mass/ volume
mass of the liquid = 21.0 g
density of the liquid = 0.865 g/ mole
volume of liquid = mass / density
= 21.0 g / 0.865g/ mole
= 24.27 ml
The total volume of the solid and liquid together is 91.0 ml.
Total volume = volume of solid + volume of liquid
volume of solid = total volume - volume of liquid
= 91.0ml - 24.27 ml
= 66.73 ml
density of the solid= 1.75 g/ mole.
mass = density * volume
= 1.75 g /mole * 66.73 ml
= 116.77 g
To learn more about Density please visit:
https://brainly.com/question/1354972
#SPJ4
I need help ASAP
What is the result of the following calculation? Record to the correct number of significant figures.
7.520 mL + 0.01509 mL = ?
a. 7.535 mL
b. 7.53 mL
c. 7.5351 ml
d. 7.53509 mL
Answers
Answer:
b
Explanation:
usually the correct number of significant figures I'd 3 so that's what I did
The result of the following calculation is 7.520 ml + 0.01509 ml = 7.53509 ml. The correct option is d.
What are calculations?Calculations are to determine mathematical problems by using the formulas of math. The basic calculation formulas that are used are addition, subtraction, multiplication, and division.
Here, is the formula that is used in addition. The sign of addition is +. The values are given in addition, we add the numbers that are given and then make the result. These given values are in decimals. So when adding the values in decimals, we put the digits down the digits and the decimals are written over the decimals.
The calculation may need a pen, paper, or calculator. Or some people do the calculations in their minds.
Thus, the correct option is d. 7.53509 mL.
To learn more about calculations, refer to the link:
https://brainly.com/question/2339329
#SPJ2
Which combination of aqueous solutions should produce a precipitate?
Question 6 options:
a) NH4F and K2CO3
b) MgCl2 and (NH4)2CO3
c) LiNO3 and Mg(CH3COO)2
d) Ba(NO3)2 and NaCH3COO
e) Na2CO3 and KOH
Answers
The combination of aqueous solutions that should produce a precipitate is the correct option b) MgCl2 and (NH4)2CO3.
This combination of aqueous solutions should produce a precipitate because when magnesium chloride (MgCl2) reacts with ammonium carbonate ((NH4)2CO3), it forms magnesium carbonate (MgCO3), which is an insoluble precipitate, along with ammonium chloride (NH4Cl), which remains dissolved in the solution.
This mixture of aqueous solutions ought to result in a precipitate because ammonium carbonate (NH4)2CO3 and magnesium chloride (MgCl2) react to form magnesium carbonate (MgCO3), an insoluble precipitate, and ammonium chloride (NH4Cl), which stays dissolved in the solution. Because of this, option (b) MgCl2 and (NH4)2CO3 is the combination of aqueous solutions that should precipitate.
Hence, The combination of aqueous solutions that should produce a precipitate is the correct option b) MgCl2 and (NH4)2CO3.
To know more about aqueous solutions click here:
https://brainly.com/question/31261482
#SPJ11