What happens to gas molecules as the pressure is decreased while the temperature and volume of the container remain constant, according to kinetic molecular theory? The frequency and force of collisions between gas molecules and the container walls decreases. The average kinetic energy of the molecules increases The attractive forces between gas molecules decrease The root mean square speed of gas molecules decreases
Answers
The Kinetic Molecular Theory, when the pressure is decreased while the temperature and volume of the container remain constant, the frequency and force of collisions between gas molecules and the container walls decreases.
Kinetic molecular theory is a theory that describes the behavior of gases. It is based on the assumption that gases consist of a large number of tiny particles that are in random motion. According to the Kinetic Molecular Theory, the following is true about gases: Gases consist of a large number of tiny particles that are in random motion.
The collisions between particles and the container walls are perfectly elastic, meaning that there is no loss of energy during the collisions. The particles are not attracted to each other, and there are no repulsive forces between them. When the pressure is decreased while the temperature and volume of the container remain constant, the frequency and force of collisions between gas molecules and the container walls decreases.
To know more about temperature visit:
https://brainly.com/question/15520591
#SPJ11
Related Questions
Use kinetic-molecular theory to describe what happens when an ice cube or icicle melts. Include how energy is involved and what happens to the motion of the water molecules
Answers
Answer and Explanation:
The Kinetic-molecular theory describes the behavior of matter that represents the movement of the tiny particles which we called as a molecules i.e. a atoms which is in the group in a given ratio
The molecules which are in the solids i.e. ice cubes are very near to each other and contains a movement that is very limited. Also their translation speed is zero but the forces i.e. intermolecular is high
In the case when a solid melts i.e. the cubes of an ice convert into a water so the molecules should be separate due to which the forces of intermolecular reduced. If the forces of intermolecular is high so it is sufficient to prevent the molecular movement in an independent way
The figure above shows two closed containers. Each contains the same volume of acetone in equilibrium with its
Yanar at the same temperature. The vapor pressure of the acetone is
Answers
The vapor pressure of a liquid and the prevailing atmospheric pressure,
determines the boiling point temperature.
The vapor pressure of the acetone is the same in both containers because
the temperature is the same.
Reasons:
The vapor pressure of a liquid is the pressure at which the number of
molecules entering the gaseous phase are in (dynamic) equilibrium with
the number of molecules entering the liquid phase.
The factors that affect vapor pressure are;
The type of molecules that make up the liquid or solidThe temperature of the liquid or solidChanging the surface area changes the instantaneous concentration of
molecules of vapor above the liquid, however, an equilibrium vapor
pressure is reached with time as more molecules enter or exits the liquid
(or solid) due being over or under saturated.
Therefore, the surface area of the liquid has no effect on a liquid's vapor
pressure therefore;
The vapor pressure in both containers will be the same because the temperature is the same.
Learn more here:
https://brainly.com/question/15171390
https://brainly.com/question/20301336

What is Ką for H3BO3(aq) = H+(aq) + H2B03 (aq)?
Answers
Answer : The expression for acid dissociation constant will be:
\(K_a=\frac{[H^+][H_2BO_3^-]}{[H_3BO_3]}\)
Explanation :
Acid dissociation constant : It is an equilibrium constant that refers to the dissociation or ionization of an acid.
It is denoted as \(K_a\).
The given equilibrium reaction is:
\(H_3BO_3(aq)\rightleftharpoons H^+(aq)+H_2BO_3^-(aq)\)
The expression for acid dissociation constant will be:
\(K_a=\frac{[H^+][H_2BO_3^-]}{[H_3BO_3]}\)
the expression for acid dissociation constant:

how many grams of na2co3 (fm 105.99) should be mixed with 5.00 g of nahco3 (fm 84.01) to produce 100 ml of buffer with ph 10.00?
Answers
2.97 grams of Na2CO₃ should be mixed with 5.00 grams of NaHCO₃ to produce 100 ml of buffer with pH 10.00.
To prepare a buffer with pH 10.00, we need to mix sodium carbonate (Na₂CO₃) and sodium bicarbonate (NaHCO₃) in the appropriate ratio to obtain the desired pH.
The pH of the buffer can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where pKa is the dissociation constant of the weak acid in the buffer (in this case, carbonic acid, H₂CO₃), [A-] is the concentration of the conjugate base (in this case, the carbonate ion, CO₃²⁻), and [HA] is the concentration of the weak acid (in this case, the bicarbonate ion, HCO³⁻).
At pH 10.00, the pKa of carbonic acid is approximately 10.33. Therefore, we can use the Henderson-Hasselbalch equation to determine the ratio of [A-]/[HA]:
10.00 = 10.33 + log([CO₃²⁻]/[HCO³⁻])
-0.33 = log([CO₃²⁻]/[HCO³⁻])
10^(-0.33) = [CO₃²⁻]/[HCO³⁻]
0.47 = [CO₃²⁻]/[HCO³⁻]
Since we are given the mass of NaHCO₃ (5.00 g), we can use its molar mass (84.01 g/mol) and the desired concentration of the buffer (100 ml) to calculate the concentration of NaHCO₃:
molar mass of NaHCO₃ = 84.01 g/mol
moles of NaHCO₃ = 5.00 g / 84.01 g/mol = 0.0595 mol
volume of buffer = 100 ml = 0.1 L
concentration of NaHCO₃ = moles / volume = 0.595 M
We can then use the equation [CO₃²⁻]/[HCO³⁻] = 0.47 to determine the concentration of Na2CO3 needed to prepare the buffer:
0.47 = [Na₂CO₃] / [NaHCO₃]
[Na₂CO₃] = 0.47 * [NaHCO₃] = 0.47 * 0.595 M = 0.28 M
Finally, we can use the molar concentration of Na₂CO₃ and the desired volume of the buffer (100 ml) to calculate the mass of Na₂CO₃ needed:
molar mass of Na₂CO₃ = 105.99 g/mol
moles of Na₂CO₃ = concentration * volume = 0.28 M * 0.1 L = 0.028 mol
mass of Na₂CO₃ = moles * molar mass = 0.028 mol * 105.99 g/mol = 2.97 g
Therefore, 2.97 grams of Na₂CO₃ should be mixed with 5.00 grams of NaHCO₃ to produce 100 ml of buffer with pH 10.00.
Know more about buffer here: https://brainly.com/question/22821585
#SPJ4
Which variable is unknown until the experiment is performed?
Answers
The variable that is unknown until the experiment is performed is the dependent variable.
In a scientific experiment, variables are classified into two main categories: independent variables and dependent variables. The independent variable is the variable that is intentionally manipulated or changed by the experimenter. It is under the control of the experimenter and is deliberately altered to observe its effect on the dependent variable.
On the other hand, the dependent variable is the variable that is measured or observed as the outcome or response in the experiment. It is the variable that is expected to change in response to the manipulation of the independent variable. The value or behavior of the dependent variable depends on the value or behavior of the independent variable.
Typically, before conducting an experiment, researchers have a hypothesis or an expectation about how the independent variable will affect the dependent variable. However, the actual outcome or result of the experiment, which is observed through the measurement of the dependent variable, remains unknown until the experiment is performed.
The purpose of conducting the experiment is to gather empirical data and observe the changes in the dependent variable to analyze the relationship between the independent and dependent variables.
For more such questions on dependent variable visit:
https://brainly.com/question/28433016
#SPJ8
difference between metals and non-metals with the reference to :
A) Number of electrons in outer or valent shell
B) Formation of cation and anion
C) Reaction with dilute action
Answers
Answer:
C) Reaction with dilute action
Explanation:
Metals are good conductors of electricity and heat.
Non-metals are insulators that don't allow heat and electricity to pass through them. Hence, non-metals are bad conductors of electricity and heat.
what is the best website to find o level chemistry notes
Answers
The finest website for you may rely on your individual requirements and interests. There are several websites that provide O level chemistry notes.
Where can I find chemistry notes online?For students seeking free access to top-notch online resources for basic, organic, inorganic, and physical chemistry notes, check ChemistNotes.com. You can get all of the Organic & Inorganic Chemistry Notes, from basic notes to advanced level notes, in one location.
What is the best method for taking notes in chemistry?An efficient and successful technique to take notes for the sciences is to use the Cornell Note-taking System. Your paper should be divided into two columns in general. You can easily review for tests using this technique, keep your notes organised, and swiftly sum up a lecture.
To know more about chemistry visit:-
https://brainly.com/question/14387251
#SPJ1
please help me out with this

Answers
2- 13.24
3- 16.18
what does a cell use for genetic material?
Answers
Answer:
Molecular genetics emerged from the realization that DNA and RNA constitute the genetic material of all living organisms. (1) DNA, located in the cell nucleus, is made up of nucleotides that contain the bases adenine (A), thymine (T), guanine (G), and cytosine (C).
blood test indicates the presence of a particular disease 93% of the time when the disease is actually present. The same test indicates the presence of the disease 0.4% of the time when the disease is not present. Three percent of the population actually has the disease. Calculate the probability that a person has the disease given that the test indicates the presence of the disease. Give your answer in decimal form, rounding to four decimal places.
Answers
The probability that a person has the disease given that the test indicates the presence of the disease is approximately 0.9968
To calculate the probability that a person has the disease given that the test indicates the presence of the disease, we can use Bayes' theorem.
Let's denote:
A = Event of having the disease
B = Event of the test indicating the presence of the disease
We are given the following probabilities:
P(A) = 0.03 (3% of the population actually has the disease)
P(B|A) = 0.93 (the test indicates the presence of the disease 93% of the time when the disease is actually present)
P(B|not A) = 0.004 (the test indicates the presence of the disease 0.4% of the time when the disease is not present)
We need to find P(A|B), the probability that a person has the disease given that the test indicates the presence of the disease.
Applying Bayes' theorem:
\(P(A|B) = (P(B|A) * P(A)) / P(B)\)
To calculate P(B), we can use the law of total probability:
\(P(B) = P(B|A) * P(A) + P(B|not A) * P(not A)\)
\(P(not A) = 1 - P(A) = 1 - 0.03 = 0.97\)
Substituting the values into the equation:
\(P(B) = (0.93 * 0.03) + (0.004 * 0.97) ≈ 0.0279\)
Now, calculating P(A|B):
\(P(A|B) = (0.93 * 0.03) / 0.0279 ≈ 0.9968\)
Therefore, the probability that a person has the disease given that the test indicates the presence of the disease is approximately 0.9968 (rounded to four decimal places).
Learn more about disease from below link
https://brainly.com/question/1268202
#SPJ11
State what would most likely happen to the rate of the enzyme action if the temperature
were reduced by 10 degrees?
Answers
Answer:
The reaction will slow down because it is below the optimal temperature.
Explanation:
Enzymes catalyses a reaction and thus increases its rate. The rate of enzyme action is dependant on temperature by Arrhenius equation. If the temperature reduces 10 degree celsius, then the rate enzyme action rate will also decreases accordingly.
What are enzymes?An enzyme is a biological catalyst. They increases the rate of chemical reactions by attaining equilibrium fastly. This is achieved by decreasing the activation energies of the reactants.
There are various kinds of enzymes which are specific in their function and specific to some substrates. The temperature at which an enzyme shows its maximum activity is called optimum temperature.
The Arrhenius equation showing the relation between rate of reaction and temperature is:
\(K = Ae^{- \frac{E_{a}}{RT} }\)
Hence, as the temperature increases rate also increase. The enzyme activity also. Therefore if the temperature reduces the enzyme activity reduces.
To find more on enzymes, refer here:
https://brainly.com/question/14953274
#SPJ2
Topic: Mass Balance. A company sells fishmeal to be used as a protein supplement in certain foods. The process consists of: a. Extraction of fish oil, stage in which a pasta is obtained that has 20% flour and 80% water. b. Drying of pasta in a rotary drum, which produces fishmeal with 40% humidity. How much pasta must be input to the process to produce 1000 kg ?
Answers
To produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta. To determine the amount of pasta required to produce 1000 kg of fishmeal, we need to consider the mass balance of the process. Let's break down the steps involved:
A. Extraction of fish oil:
The pasta obtained from the extraction stage contains 20% flour and 80% water. To calculate the amount of pasta, we need to determine the mass of flour and water in the pasta. Let's assume the total mass of the pasta is P kg.
Mass of flour = 20% of P = 0.2P kg
Mass of water = 80% of P = 0.8P kg
b. Drying of pasta:
During the drying stage, the pasta is dried in a rotary drum, resulting in fishmeal with 40% humidity. This means that the final fishmeal will contain 60% dry matter.
Let's assume the mass of the dried fishmeal is M kg.
Mass of dry matter = 60% of M = 0.6M kg
Since the dry matter in the fishmeal comes from the flour in the pasta, we can equate the mass of dry matter to the mass of flour:
0.6M kg = 0.2P kg
To produce 1000 kg of fishmeal, we want to find the corresponding value of P:
0.6M = 0.2P
P = (0.6M) / 0.2
P = 3M
Therefore, to produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta.
To know more about mass balance, click here, https://brainly.com/question/17014679
#SPJ11
Hexachlorobenzene is contaminating your local well water. The concentration in the ground water is 3.4 mg/L. The soil organic content is 0.3% and pore water occupies 55% of aquifer volume. Estimate carbon-normalized sorption coefficient, sorption coefficient and mass of organic contaminant per mass of sorbent.
Answers
To estimate the carbon-normalized sorption coefficient, sorption coefficient, and mass of organic contaminant per mass of sorbent, we need additional information such as the organic carbon partition coefficient (Koc) and the bulk density of the soil.
Carbon-Normalized Sorption Coefficient (Koc):
The carbon-normalized sorption coefficient represents the sorption capacity of the sorbent material for the organic contaminant. It is calculated by dividing the sorption coefficient (Kd) by the organic carbon content in the soil.
Koc = Kd / % organic carbon
Sorption Coefficient (Kd):
The sorption coefficient represents the ratio of the concentration of the contaminant adsorbed onto the sorbent material to the concentration in the aqueous phase.
Kd = (mass of contaminant sorbed / mass of sorbent) / (concentration of contaminant in water)
Mass of Organic Contaminant per Mass of Sorbent:
To calculate the mass of organic contaminant per mass of sorbent, we need to know the mass of the contaminant adsorbed onto the sorbent material and the mass of the sorbent itself.
Mass of organic contaminant per mass of sorbent = mass of contaminant sorbed / mass of sorbent
To perform these calculations, we need the organic carbon partition coefficient (Koc) and the bulk density of the soil. With this information, we can estimate the sorption characteristics of hexachlorobenzene in the given scenario.
Learn more about soil contamination here: brainly.com/question/32841304
#SPJ11
covalent compounds contain
Answers
Answer: Covalent compounds have bonds where electrons are shared between atoms. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower melting points and electrical conductivity compared to ionic compounds.
hope this helps!
Explanation:
Which has more total Heat Energy/
A.) A cup of HOT coffee
B.) A thermos of HOT coffee
C.) They both have the same amount to heat energy
Answers
Answer:
The thermos should have more heat energy because of the greater mass.
You come across a mysterious substance that has a mass of 1.8225 x 10 to the power of 2g
and a volume of 0.025 L (1L = 1000 mL). Using the density formula what is the density
Answers
Answer:
192
Explanation:
Bc its 182
Please help! I have 2 remaining attempts and only 2 of my answers are incorrect. Please let me know which reactions are wrong and where they should be put! LOTS OF POINTS!!!!
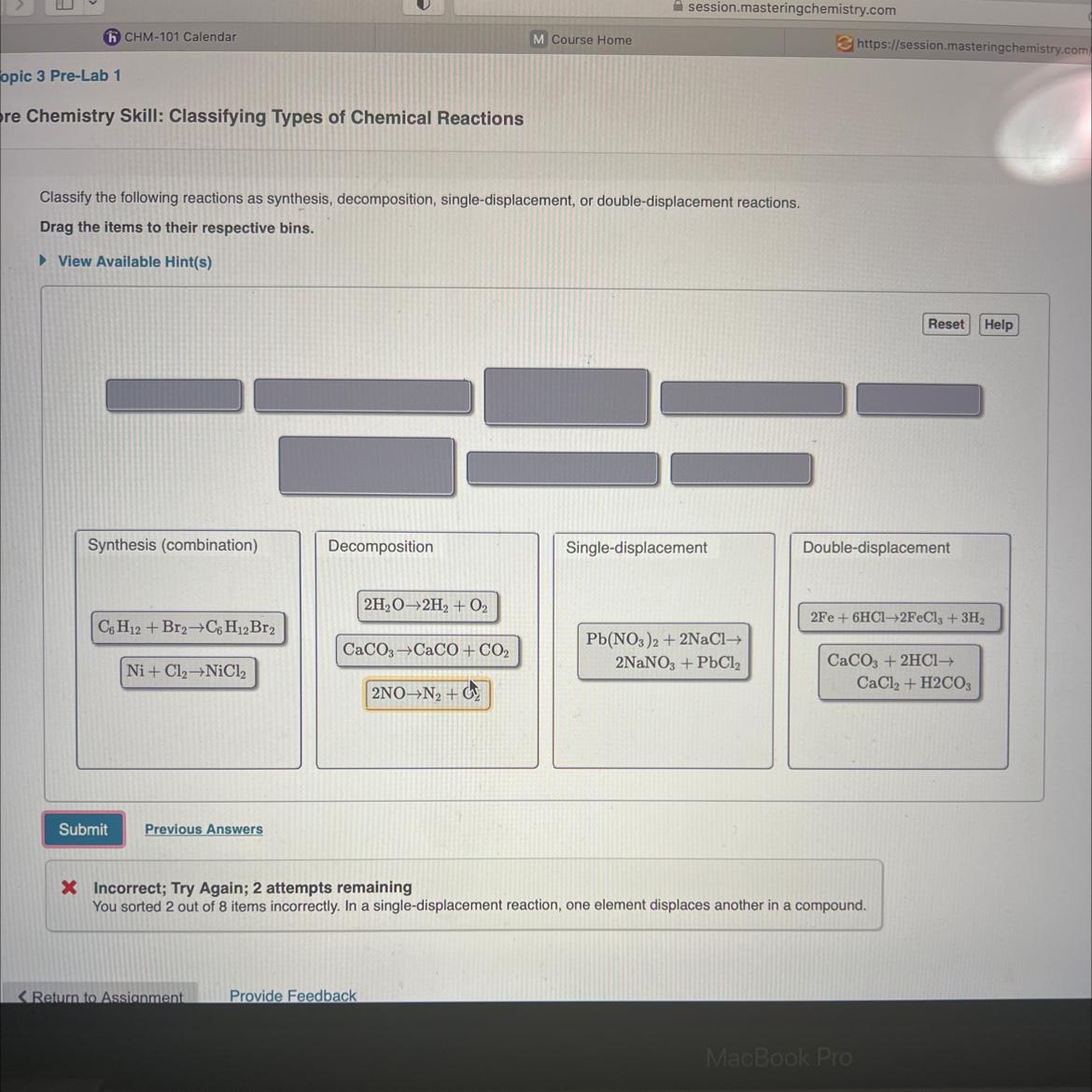
Answers
I think the second one needs to be put on the sixth area.
Base your answer on the information belowWhich gas occupies the smallest volumeA. BB. DC. CD. A

Answers
The question requires us to chose among gases A, B, C and D which one would present the smallest volume, given the number of moles, pressure and temperature of each gas.
The ideal gas equation relates the pressure, volume, number of moles and temperature of an ideal gas as it follows:
\(P\times V=n\times R\times T\)where P is the pressure of the gas, V is its volume, n corresponds to the number of moles of gas, T to the temperature and R is the constant of gases.
We can rearrange the equation above to look specifically at the volume of the gas:
\(V=\frac{n\times R\times T}{P}\)We can note from the equation above that the volume of a gas should increase with its number of moles and temperature (directly proportional), and decrease with the increase of pressure (inversely proportional). Based on this, we could infer that the gas with smallest pressure would present the biggest volume - but, as the question provided values of n and T and they are not the same for all gases, it is not a completely correct affirmation in this case.
Since the question provided the values of n, P and T for all samples, we can calculate the volume of each gas using the equation above and determine which one would present the smallest volume. Not that, as R is a constant and won't change, we don't need to add it to the calculation (we'll keep it as the variable R and it won't affect the values if they are only considered to compare the volume of gases):
\(\begin{gathered} V_A=\frac{(2\text{mol)}\times(273K)\times R}{\mleft(760\operatorname{mm}\mright)}=0.718R \\ \\ V_B=\frac{(1\text{mol)}\times(273K)\times R}{(380\operatorname{mm})}=0.718R \\ \\ V_C=\frac{(1\text{mol)}\times(273K)\times R}{(760\operatorname{mm})}=0.359R \\ \\ V_D=\frac{(2\text{mol)}\times(546K)\times R}{(760\operatorname{mm})}=1.437R \end{gathered}\)Therefore, considering the information given, the gas with smallest volume would be gas C and the best option to answer the question is letter C.
Calculate the volume in L of 11.6 moles of Neon at 120 K when it has a pressure of 25.9 atm
Answers
Answer:
The volume of the gas is approximately 4.41 liters
Explanation:
The details of the data of the Neon gas are;
The number of moles of Neon gas present, n = 11.6 moles
The temperature of the sample of Neon gas, T = 120 K
The pressure of the sample of the Neon gas, P = 25.6 atm
By the ideal gas equation, we have;
P·V = n·R·T
Where;
R = The universal gal constant = 0.08205 L·atm·mol⁻¹·K⁻¹
Therefore, we get;
V = n·R·T/P
Which gives;
V = 11.6 moles × 0.08205 L·atm·mol⁻¹·K⁻¹ × 120 K/(25.9 atm) ≈ 4.4097915 L
The volume of the gas, V ≈ 4.41 L.
Give one example of each of the following, that happens to us in our everyday life: Explain a bit about the science behind it, so for example, for melting you can say ice cream melting in your hand, which turns from a solid to a liquid, which is melting. If you are unsure please do not answer, though if you are confident please be free to do so! Have a wonderful day or night!
a) Melting:
b) Freezing:
c) Condensation:
d) Evaporation:
e) Sublimation.
Answers
a) Melting: An example of melting that occurs in our everyday life is when we heat butter on a stovetop.
b) Freezing: Freezing is the process in which a liquid transforms into a solid upon cooling.
c) Condensation: One example of condensation that we encounter regularly is when water droplets form on the surface of a cold drink on a hot day.
d) Evaporation: Evaporation is the process by which a liquid transforms into a gas or vapor.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state.
a) Melting: Butter is a solid at room temperature, but when heat is applied, it melts into a liquid. This change is a result of the increase in temperature, which provides enough energy to overcome the intermolecular forces holding the butter molecules together.
b) Freezing:Eventually, the temperature reaches the freezing point of water (0°C or 32°F), at which the water molecules slow down and arrange themselves into a regular, crystalline structure. This transformation from a liquid to a solid state is accompanied by the release of heat energy.
c) Condensation: As the temperature decreases, the air's capacity to hold moisture decreases, causing the water vapor in the air to condense into liquid water droplets. This process occurs due to the transfer of heat energy from the warm air to the cold surface, leading to the saturation of the air and the conversion of water vapor into liquid form.
d) Evaporation: As the sun's heat energy is absorbed by the water molecules on the clothes' surface, their kinetic energy increases, causing them to break free from the liquid phase and escape into the surrounding air as water vapor. This process occurs because the molecules at the liquid surface with sufficient energy can overcome the attractive forces within the liquid and enter the gas phase.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state. An example of sublimation is the process of dry ice (solid carbon dioxide) converting into carbon dioxide gas.
For more such questions on Freezing visit:
https://brainly.com/question/40140
#SPJ8
Assertion: When a strong acid is added to a buffer system consisting of a weak acid (HA) and its conjugate base (A-), the concentration of the conjugate base increases. Reason: A stoichiometric amount of the weak acid is converted to its conjugate base. Group of answer choices
Answers
Answer:
Both the assertion and reason are false
Explanation:
A buffer is a solution that resists changes in acidity and alkalinity. When a solution is buffered, it pH can only vary within a small range. A buffer is made up of a weak acid/base and its salt.
When a strong acid is added to a buffer solution, the conjugate base will react with the H+ from the strong acid to form the undissociated weak acid HA as follows; H+(aq) + A- (aq)→ HA(aq). Hence, H+ concentration decreases owing to its reaction with the A- thus the pH changes only slightly.
At the time when the strong acid should be added so here Both the assertion and reason are false
What is buffer?It is a solution that resists changes with respect to the acidity and alkalinity. When a solution should be buffered, it pH can only change within a small range. At the time when a strong acid should be added to a buffer solution, the conjugate base should be react with the H+ from the strong acid to form the undissociated weak acid.
Learn more about buffer here: https://brainly.com/question/13861408?referrer=searchResults
The air in the balloon i heated up by leaving it in a warm place. Give two effect that thi ha on the air particle
Answers
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
What is pressure?
Pressure is the force applied perpendicular to the surface of an object per unit area over which that force is distributed.
Various units are used to express pressure. Some of these are units of force divided by units of area. For example, the SI unit of pressure, Pascal (Pa), is 1 Newton per square meter (N/m2). Similarly, pounds force per square inch (psi, symbol lbf/in2) is the traditional unit of pressure in imperial and US systems. Pressure can also be expressed as standard atmospheric pressure. Atmospheric pressure (atm) is equal to this pressure and torr is defined as 1/760 of this. Manometric units such as centimeters of water, millimeters of mercury, and inches of mercury are used to express pressure as the height of a particular liquid column within a manometer.
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
To know more about Pressure, visit:
https://brainly.com/question/28012687
#SPJ4
Which molecule in the net reaction of the citrate cycle contributes to the inhibition of pyruvate dehydrogenase?a. NADH.
b. H+.
c. H2O.
d. FAD.
Answers
NADH is the substance that aids in the citric acid cycle's inhibition of pyruvate dehydrogenase.
An enzyme called pyruvate dehydrogenase catalyzes the transformation of pyruvate into acetyl-CoA, which serves as the starting point for the citric acid cycle. The accumulation of NADH, a byproduct of the citric acid cycle, can inform the cell that its energy requirements have been satisfied.
By attaching to a particular regulatory site on pyruvate dehydrogenase, NADH functions as an allosteric inhibitor of the enzyme, reducing its activity and slowing the rate of the citric acid cycle. This aids in controlling the cell's production of acetyl-CoA and preventing the overproduction of ATP.
To Learn More About citric acid cycle Click
https://brainly.com/question/29857075
#SPJ4
Cho biết X, Y (ZX < ZY) là hai nguyên tố mà lớp vỏ nguyên tử có electron cuối cùng điền vào phân lớp 4s.
a/ Viết cấu hình electron của X, Y.
b/ Viết kí hiệu nguyên tử của X, Y.
Answers
Select the correct answer.
In the following reaction, how many liters of O₂ will produce 43,62 liters of CO₂ at STP?
CaHa +50₂
3 CO₂ + 4H₂O
OA. 72.7 liters
OB.
OC.
OD.
E.
17.45 liters
14.54 liters
54.53 liters
36.3 liters
Answers
In the subsequent reaction, at STP, 72.7 litres of oxygen will result in 43,62 litres of carbon dioxide.
How is sulphur IV oxide written?How sulfur(IV) oxide, or SO2, is made and its properties. Sulfur(IV) oxide is also referred to as sulphur dioxide in everyday speech. It is a chemical compound with the formula SO2 whose molecule is made up of one sulphur atom and two oxygen atoms.
C3H8 + 5O2 → 3CO2 + 4H2O
According to the balanced equation, 3 moles of CO2 are produced for every 5 moles of O2 used. Therefore, we can use the following proportion to find the volume of O2 required to produce 43.62 liters of CO2 at STP:
5 L O2 / 3 mol CO2 = x L O2 / 43.62 L CO2
We need to first find the number of moles of CO2 produced
43.62 L CO2 × 1 mol CO2 / 22.4 L = 1.95 mol CO2
Using the balanced equation, we can see that 5 moles of O2 are required to produce 3 moles of CO2.
1.95 mol CO2 × 5 mol O2 / 3 mol CO2 = 3.25 mol O2
Finally, we can use the ideal gas law to find the volume of O2 required at STP:
PV = nRT
V = nRT / P
V = (3.25 mol)(0.0821 L·atm/K·mol)(273 K) / (1 atm) = 72.7 L
To know more about carbon dioxide visit:-
https://brainly.com/question/3049557
#SPJ1
Round the measurement to 3 significant figures. 13.06
Answers
Answer: 13.1
Explanation: just round the .06 to 1 creating three sig figs.
What are the two types of carbohydrates and food sources?.
Answers
Answer: simple and complex.
Explanation:These are also called simple sugars. They're found in refined sugars, like the white sugar you see in a sugar bowl. If you have a lollipop, you're eating simple carbs.
how many grams of ammonium carbonate (96.09 g/mol) should be added to 438 ml of 0.18 m of ammonium nitrate in order to produce an aqueous 0.67 m solution of ammonium ions? enter your answer to 2 decimal places.
Answers
Therefore, approximately 22.61 grams of ammonium carbonate should be added to 438 mL of 0.18 M ammonium nitrate solution to produce an aqueous 0.67 M solution of ammonium ions.
The balanced equation for the reaction between ammonium carbonate (NH4)2CO3 and ammonium nitrate NH4NO3 is:
(NH4)2CO3 + NH4NO3 -> 2NH4+ + CO3^2- + NO3^-
From the balanced equation, we can see that one mole of (NH4)2CO3 produces 2 moles of NH4+ ions.
Given:
Volume of ammonium nitrate solution = 438 mL = 0.438 L
Molarity of ammonium nitrate solution = 0.18 M
Desired molarity of ammonium ions = 0.67 M
Molar mass of ammonium carbonate = 96.09 g/mol
Calculate the moles of ammonium nitrate:
Moles of NH4NO3 = Molarity × Volume
Moles of NH4NO3 = 0.18 M × 0.438 L
Calculate the moles of ammonium ions:
Moles of NH4+ = Moles of NH4NO3 × 2
Calculate the volume of ammonium carbonate solution required:
Volume of (NH4)2CO3 solution = Moles of NH4+ / Desired molarity of NH4+
Calculate the mass of ammonium carbonate:
Mass of (NH4)2CO3 = Volume of (NH4)2CO3 solution × Molarity × Molar mass
Let's perform the calculations:
Moles of NH4NO3 = 0.18 M × 0.438 L = 0.07884 mol NH4NO3
Moles of NH4+ = 0.07884 mol NH4NO3 × 2 = 0.15768 mol NH4+
Volume of (NH4)2CO3 solution = 0.15768 mol NH4+ / 0.67 M = 0.23546 L
Mass of (NH4)2CO3 = 0.23546 L × 96.09 g/mol = 22.61 g
Learn more about ammonium nitrate solution here
https://brainly.com/question/5148461
#SPJ11
The word equation for rusting is shown below. What word should go in the first gap?
iron + oxygen + water → _____1_____ _____2_____ (__3__) _____4_____
Answers
The complete word equation for the rusting of iron is; iron + oxygen + water → hydrated Iron III oxide.
What is rusting?The term rusting has to do with the process by which iron reacts with moisture in the presence of oxygen and forms a flaky substance that reduces the tensile strength of the iron. We know that the process of rusting is an example of an unwanted oxidation reaction and this is why the iron must be protected from rusting.
Thus, the reactants that we can find in the rusting of iron are iron metal, water and oxygen. This is a chemical change because the compound that is produced is quite different in terms of chemical composition from the reactants.
Learn more about rusting:https://brainly.com/question/18376414
#SPJ1
PLS HELP!!!!! WILL GIVE BRAINLIEST, 5 STARS, AND THANKS!!!!!!!!!!
Which reactions are the reverse of one another?
condensation and addition
addition and elimination
elimination and condensation
substitution and addition
Answers
Answer:
it has to be b
Explanation:
the other ones don't make any sense