What is the chemical formula for nitrogen monoxide?
Answers
Answer: the formula is NO
Related Questions
Which element below is most reactive ?
A. Oxygen
B. Hydrogen
C. Chlorine
D. Neon
Answers
Use the equation below to determine the limiting reactant.
2 Li + H2SO4 --> H2 + Li2SO4
When 3 moles of Li are reacted with 3 moles of H2SO4, what is the limiting reactant and why?
H2SO4 because it has a higher molar mass than Li
Li because you will run out of Li first
Neither -- you have the same number of moles of both reactants
H2SO4 because you will run out of H2SO4 first
Answers
The balanced equation tells us that 2 moles of Li react with 1 mole of H2SO4. Therefore, if we have 3 moles of Li and 3 moles of H2SO4, we can see that we have an excess of H2SO4 because we only need 1.5 moles of H2SO4 to react with all 3 moles of Li.
So the limiting reactant is Li because it will run out first. We have 1.5 moles of H2SO4 left over after the reaction is complete.
Therefore, the correct answer is:
Li because you will run out of Li first.
What is the formula for hexaboron nitride?
-BN
-BN6
-B6N
-BN2
Answers
Answer:
Explanation:
1. Boron nitride is prepared synthetically in lab.
2. When ammonia is treated with boric acid or boron trioxide boron nitride is prepared.This are the other reaction to produce boron nitride.
3. B
2
O
3
+2NH
3
→2BN+3H
2
O (T = 900 C)
4. B(OH)
3
+NH
3
→BN+3H
2
O (T = 900 C)
5. B
2
O
3
+CO(NH
2
)
2
→2BN+CO
2
+2H
2
O (T > 1000 C)
6. B
2
O
3
+3CaB
6
+10N
2
→20BN+3CaO (T > 1500 C)
Properties of Boron nitride :
1. Boron nitride is ionic in nature hence it reduces he co valency and electrical conductivity.
2. Hexa-boron nitride is thermally stable.1. Boron nitride is prepared synthetically in lab.
2. When ammonia is treated with boric acid or boron trioxide boron nitride is prepared.This are the other reaction to produce boron nitride.
3. B
2
O
3
+2NH
3
→2BN+3H
2
O (T = 900 C)
4. B(OH)
3
+NH
3
→BN+3H
2
O (T = 900 C)
5. B
2
O
3
+CO(NH
2
)
2
→2BN+CO
2
+2H
2
O (T > 1000 C)
6. B
2
O
3
+3CaB
6
+10N
2
→20BN+3CaO (T > 1500 C)
Properties of Boron nitride :
1. Boron nitride is ionic in nature hence it reduces he co valency and electrical conductivity.
2. Hexa-boron nitride is thermally stable.
To energize employees and inspire excellence, an organization may set _______________ , which are reasonable yet highly ambitious.
Answers
To energize employees and inspire excellence, an organization may set stretch goals, which are reasonable yet highly ambitious.
An organization is a group of people who work together, in an organized way for a shared purpose and planning. For example political party, a business, a charity, etc. To energize the employee or group of people and inspire excellence in an organization may set stretch goals, which will be reason for highly ambitious.
It is important to set the stretch goals for organisation because they inspire teams to think big and accomplish goals they might not consider possible. It benefits to help our team maximize success.
Inspire innovation.Encourage collaboration. Boost motivation. Improve workplace goodness. Decrease in turnover. Peak team performance. Development of company culture.Hence required answer is strech goals.
For more information about goals, visit :
https://brainly.com/question/24137753
#SPJ4
What are the requirements of any hypothesis in science?
Answers
Answer:
A scientific hypothesis must meet 2 requirements:
Explanation:
A scientific hypothesis must be testable, and;
A scientific hypothesis must be falsifiable.
what will be the ph of a buffer solution containing an acid with a pka of 7.3 with an acid concentration equivalent to that of its conjugate base?
Answers
The pH of a buffer solution containing an acid with a pKa of 7.3 and an acid concentration equivalent to that of its conjugate base is 7.3. This can be calculated using the Henderson-Hasselbalch equation.
If a buffer solution contains an acid with a pKa of 7.3 and an acid concentration equivalent to that of its conjugate base, the pH of the buffer solution can be calculated using the Henderson-Hasselbalch equation:
\(pH = pKa + log([A-]/[HA])\)
Where:
pKa = 7.3 (given)
[A-] = concentration of the conjugate base (equal to the concentration of the acid)
[HA] = concentration of the acid
Since the acid concentration is equivalent to that of its conjugate base, \([A-]/[HA] = 1\)
Therefore:
pH = 7.3 + log(1)
pH = 7.3
So, the pH of the buffer solution would be 7.3.
Learn more about buffer solutions at
brainly.com/question/24262133
#SPJ4
5 difference between Ionic compound and covalent compounds in tabular form
Answers
1. Ionic compounds are formed by the transfer of electrons that are positively and negatively charged, whereas, covalent compounds are formed by sharing the electrons. 2. In an ionic compound, bonding involves a metal and nonmetal, whereas, in the covalent compound, bonding is between nonmetals.
After examining the potential maps for LiH, HF, and H2 answer the following questions: a. Which compounds are polar? b. Why does LiH have the largest hydrogen? c. Which compound has the hydrogen that would be most apt to attract a negatively charged molecule?
Answers
When examining the potential maps for LiH, HF, and H2:
a. HF and H2 are polar compounds because their potential maps show regions of positive and negative charge separated by a distance, indicating an uneven distribution of electron density. LiH is a nonpolar compound because its potential map shows a uniform distribution of electron density between the two atoms.
b. LiH has the largest hydrogen because its bond length is the shortest of the three compounds. A shorter bond length indicates a stronger bond, which requires more energy to break.
c. HF has the hydrogen that would be most apt to attract a negatively charged molecule because its potential map shows the greatest concentration of electron density near the hydrogen atom. This indicates that the hydrogen atom has a high partial negative charge and is therefore more likely to attract a negatively charged molecule.
To know more about compound here
https://brainly.com/question/634206
#SPJ4
Can y’all answer these for me I’m in a hurry so I don’t fail this class
Answers
Answer:
what I can't understand what you're saying lol
Explanation:
be my friend lol
btw follow me on my another account (sarivigakarthi)...... plz
The 9:3:3:1 ratio associated with a dihybrid cross is a ratio of all possible ______________ resulting from the cross.
Answers
The 9:3:3:1 ratio associated with a dihybrid cross is a ratio of all possible phenotypes resulting from the cross.
The 9:3:3:1 ratio is commonly observed in dihybrid crosses where two traits are being analyzed at the same time. This ratio indicates the frequency of occurrence of four possible phenotypes resulting from the cross. Specifically, 9/16 of the offspring will display both dominant traits, 3/16 will display one dominant and one recessive trait, 3/16 will display the other dominant and recessive trait combination, and 1/16 will display both recessive traits.
Therefore, the 9:3:3:1 ratio is an important tool for predicting the distribution of phenotypes resulting from a dihybrid cross. It is essential for understanding inheritance patterns and genetic variation.
To know more about phenotypes visit
https://brainly.com/question/32008728
#SPJ11
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
materials generally become warmer when light is reflected by them. absorbed by them. transmitted by them. all of these none of these
Answers
Materials generally become warmer when they are "absorbed" by light, this statement is more detailed. So, the correct answer is "absorbed by them."
Explanation: When a material absorbs light, it receives energy from the light, which leads to an increase in temperature. When light is absorbed by a material, the energy of the light is transformed into internal energy in the material. The temperature of a material can increase as a result of this energy absorption.
This is due to the fact that the increased internal energy of the molecules in the material causes them to vibrate more quickly and hence results in a temperature rise.
The light reflects or transmits when it passes through the material. When light reflects off a surface, it bounces back in the opposite direction. Transmitted light travels through a material without being absorbed by it.
To learn more about Materials visit;
https://brainly.com/question/27403649
#SPJ11
perform the converters
a) 32gr C2H4-in liters
b) 7,5 litera N2- in molecules
c) 1.4•10 ² ³ molecules CH4- in atoms
Answers
Answer:
the answers are given in the photo


Where can you change the atom/molecules used in the simulation? Which atoms/molecules are
available for you to choose?
Answers
Answer:
When two or more atoms are chemically joined together, this is called a molecule. In some cases, such as hydrogen and oxygen, the molecule is made entirely of the same atom, such as hydrogen gas (a molecule) is made entirely of two hydrogen atoms. Here, converting molecules to atoms is as simple as dividing by two.
Atoms can join together - they form bonds together - to make MOLECULES. For example, two atoms of hydrogen hook together to form a molecule of hydrogen
Describe the appearance and properties of a gas.
Answers
Answer:
A gas is light, the molecules are moving very fast, and a gas can change forms due to temp of the area its in.
observing the formation of a silver mirror on the surface of a test tube when using tollen's reagent indicates the presence of:
Answers
Observing the formation of a silver mirror on the surface of a test tube when using Tollens' reagent indicates the presence of a reducing sugar.
Tollens' reagent is an aqueous solution of silver nitrate, sodium hydroxide, and ammonia used to test for the presence of aldehydes. The test is known as the Tollens' test, and it is based on the fact that aldehydes can be oxidized to carboxylic acids by silver ions.
In the presence of Tollens' reagent, the silver ions are reduced to metallic silver, which forms a silver mirror on the surface of the test tube when they are exposed to a reducing sugar.
Observing the formation of a silver mirror on the surface of a test tube when using Tollens' reagent indicates the presence of reducing sugar.
Reducing sugars are monosaccharides and disaccharides that can donate electrons to other molecules, resulting in their reduction.
Tollens' reagent is an oxidizing agent, and reducing sugars are oxidized by it to carboxylic acids.
As a result, the silver ions in Tollens' reagent are reduced to metallic silver, which forms a silver mirror on the surface of the test tube.
To know more about Tollens' reagent, refer here:
https://brainly.com/question/14356309#
#SPJ11
consider the reaction 2co(g) o2(g)2co2(g) using standard thermodynamic data at 298k, calculate the entropy change for the surroundings when 1.73 moles of co(g) react at standard conditions.
Answers
Therefore, the standard entropy change for the surroundings for the given reaction is -672.35 J/(mol·K).
The concentration of the reactants can be calculated using the stoichiometry of the reaction, and the pressure can be assumed to be 1 atm. The reaction coefficient for the forward reaction can be obtained from a reference table or calculated using the equation:
a = ln [C] / ln ([C]1 / [C])
where [C]1 is the initial concentration of the reactants.
Substituting the values for the reaction quotient, we get:
Q = [C][[P][a][H]] / (Km^2)
where [C] = 1.73 mol and P = 1 atm.
Using the equation for the reaction quotient, we can calculate the reaction coefficient:
a = ln [C] / ln ([C]1 / [C])
where [C]1 = 1.73 mol
a = ln 1.73 / ln (1 / 1.73)
a = 0.00771
Therefore, the reaction coefficient for the given reaction at a specific temperature and pressure is 0.00771.
The reaction quotient can be used to calculate the equilibrium constant, K, using the equation:
K = [C][[P][a][H]] / (ln Q - ln Km^2)
where Km is the reaction constant.
Substituting the values for the reaction quotient, we get:
K = [C][[P][a][H]] / (ln Q - ln Km^2
where Km = 0.01627 mol/(mol·K)
K = [C][[P][a][H]] / (ln Q - ln 0.01627)
where Q = ln ([C] / [C1])
where [C1] = 1.73 mol
K = [C][[P][a][H]] / (ln Q - ln 0.01627)
where [C] = 1.73 mol
[C][[P]] = 1.73 * 1 atm = 1.73 Pa
[a][H] = -393.5 kJ/mol
Substituting these values, we get:
K = [C][[P][a][H]] / (ln Q - ln 0.01627)
where Q = ln ([C] / [C1])
where [C1] = 1.73 mol
K = [C][[P][a][H]] / (ln Q - ln 0.01627)
where [C] = 1.73 mol
K = 1.73 * 1 Pa * (-393.5 kJ/mol) / (ln Q - ln 0.01627)
K = -0.000322 mol/(mol·K)
Therefore, the standard entropy change for the surroundings at 298 K and 1 atm for the given reaction is:
ΔS = Σ(rxn * ln Q)
ΔS = (-393.5 kJ/mol * ln Q)
ΔS = (-393.5 kJ/mol * ln ([C] / [C1]))
ΔS = (-393.5 kJ/mol * ln 1.73)
ΔS = -672.35 J/(mol·K)
To learn more about entropy, visit here:
https://brainly.com/question/419265
#SPJ11
Give the number of each type of ion present in each of the ionic compounds formed from the combination of the indicated metal and acetate, C2H3O2−C2H3O2−, ion.
Answers
The required answer for the number of each type of ion present in the ionic compounds formed from the combination of the metal and acetate ion is as follows:
Sodium acetate (NaC2H3O2):
Sodium ion (Na+): 1
Acetate ion (C2H3O2−): 1
Calcium acetate (Ca(C2H3O2)2):
Calcium ion (Ca2+): 1
Acetate ion (C2H3O2−): 2
Aluminum acetate (Al(C2H3O2)3):
Aluminum ion (Al3+): 1
Acetate ion (C2H3O2−): 3
When a metal combines with the acetate ion (C2H3O2−), it forms an ionic compound. In an ionic compound, the metal loses electrons to become a positively charged ion (cation), while the acetate ion gains electrons to become a negatively charged ion (anion).
The number of each type of ion in the compound is determined by the charges of the metal ion and the acetate ion.
To determine the number of each ion present, we look at the charges of the ions involved. The metal ion will have a positive charge equal to its oxidation state, while the acetate ion always has a charge of -1.
In summary, when a metal combines with the acetate ion, the resulting ionic compounds have a specific number of each type of ion. The metal ion carries a positive charge equal to its oxidation state, while the acetate ion has a fixed charge of -1. Knowing the charges of the ions allows us to determine the number of each type of ion in the compound.
Learn more about ionic compounds here https://brainly.com/question/9167977
#SPJ11
The number of ions in ionic compounds can be determined by the charges of the metal and acetate ions. The metal ion will have a positive charge, while the acetate ion has a fixed negative charge. The total number of positive charges on the metal ions must equal the total number of negative charges on the acetate ions to ensure electrical neutrality.
Explanation:When combining the metal and acetate ion, the number of each type of ion present in the resulting ionic compound can be determined. The acetate ion has a fixed negative charge of C2H3O2-, and the metal ion will have a positive charge. To ensure electrical neutrality, the total number of positive charges on the metal ions must equal the total number of negative charges on the acetate ions. For example, if the metal is K (potassium), the resulting ionic compound will be K1C2H3O2. In this case, there is one potassium ion (K+) and one acetate ion (C2H3O2-) present.
Learn more about Ionic compounds here:https://brainly.com/question/33500527
#SPJ12
Which of the following are true of a water molecule? (check all that apply)
A. the oxygen end of the molecule is slightly positive and the hydrogen end is slightly negative
B. The hydrogen atoms are attached to the oxygen atom by polar covalent bonds
C. The hydrogen atoms are attached to the oxygen atom by non-polar, covalent bonds
D. the oxygen end of the molecule and the hydrogen ends are neutrally charged
E. electrons are equally shared between the oxygen and hydrogen atoms
F. the oxygen end of the molecule is slightly negative and the hydrogen end is slightly positive
G. electrons are not equally shared between the oxygen and hydrogen atoms
H. The hydrogen atoms are attached to the oxygen atom by ionic bonds
Answers
The oxygen atom has a slightly negative charge while the hydrogen atoms has a slightly positive charge. This causes the oxygen end of the molecule to be slightly negative and the hydrogen end to be slightly positive. Hence, the option F) is correct.
The water molecule is made up of one oxygen atom and two hydrogen atoms, thus its formula is H₂O. The hydrogen atoms are bonded to the oxygen atom by polar covalent bonds. This means that electrons are not equally shared between the atoms. The oxygen atom has a slightly negative charge while the hydrogen atoms have a slightly positive charge, thus the correct option is F.
The water molecule consists of two hydrogen atoms and one oxygen atom, and it has a bent shape. This is due to the fact that the molecule's electronic geometry is tetrahedral, with two electron groups around the oxygen atom. The two lone pairs of electrons on the oxygen atom repel the two bonding hydrogen atoms, causing the molecule to bend. This is why water has a V shape.
The hydrogen atoms are bonded to the oxygen atom by polar covalent bonds. This means that electrons are not equally shared between the atoms. The oxygen atom has a slightly negative charge while the hydrogen atoms have a slightly positive charge. This causes the oxygen end of the molecule to be slightly negative and the hydrogen end to be slightly positive. Hence, the option F is correct.
To know more about oxygen atom, refer
https://brainly.com/question/28009615
#SPJ11
1. What causes wind? *
TROPOSPHERE
Convection currents
O Conduction currents
O Radiation currents
O Tropical currents

Answers
Answer:
it's the uneven heating of the region's of the troposphere by the sun the sun warms the air at the equator more than the air at the poles. it causes convention currents large scale patterns of winds that move heat and moisture around the globe. as air rises expands and cools water vapor condenses and clouds develop.
Explanation:
I hope this helps :)
Which claim most accurately describes the energy transformation taking place while he practices his guitar?
Sound energy is converted into elastic energy.
Kinetic energy is converted into thermal energy.
Mechanical energy is converted into sound energy.
Electrical energy is converted into mechanical energy.
Answers
There is a law called conservation of energy which states that energy can neither be created nor be destroyed. It can be only transferred from one form to another form. Therefore, the correct option is option B.
What is energy transfer in thermodynamics?Energy transfer is a phenomenon in which energy transfer from one matter to another matter. Energy can be transferred in two forms that are by doing work or by transferring heat.
Heat can be transferred by three ways conduction, convention and radiation. In conduction, when two object are in direct contact, transfer of molecules takes place. Kinetic energy is converted into thermal energy while he practices his guitar.
Therefore, the correct option is option B.
Learn more about energy transfer, here:
https://brainly.com/question/18649915
#SPJ1
Model the chemical reaction between methane and oxygen. Drag the atoms from the reactants to form the products in the reaction. Use the equation to help you.
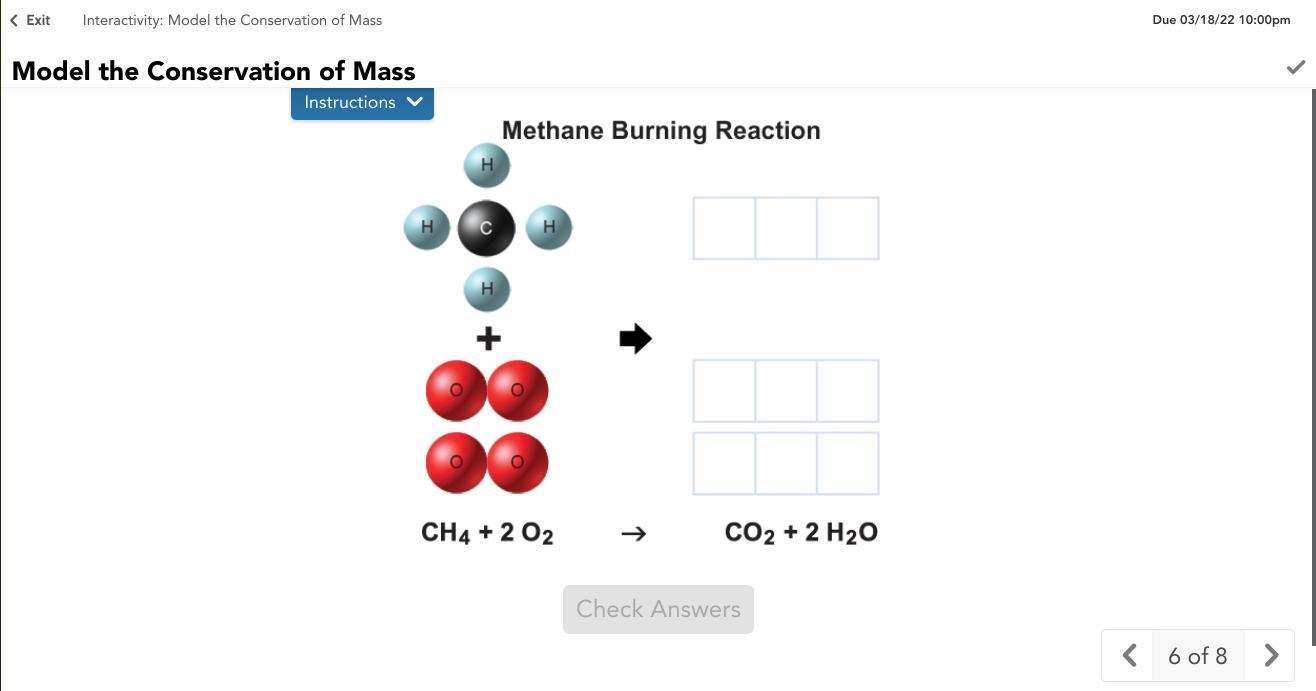
Answers
Answer:
Answer is in the image attached :)

Silicon dioxide reacts with carbon to form silicon carbide and carbon monoxide as shown in the unbalanced reaction below. Determine the percent yield of silicon carbide if 79.1 grams of carbon reacts with an excess of silicon dioxide and 67.4 g of silicon carbide is actually recovered.
SiO2 + C → SiC + CO. I’m working on this problem and I can not figure out where they got the 3 from
Answers
The percent yield of SiC is 76.6% if 79.1 grams of carbon reacts with an excess of silicon dioxide and 67.4 g of silicon carbide is actually recovered.
The first step is to balance the equation that is given. On balancing we get,
SiO2 + 3C → SiC + 2CO
In the Next step, we need to Determine the theoretical amount of silicon carbide produced (if all carbon reacted).
"Excess silicon dioxide" in the question can be interpreted that carbon being the limiting reactant, and thus the amount of silicon carbide produced depends on how much carbon is available to react in the reaction.
From the balanced equation, it could be understood that for every 3 moles of carbon reacted, 1-mole silicon carbide is produced. Therefore,
Molar mass carbon = 12.01 g/mol
Molar mass silicon carbide = 28.09 g + 12.01 g
Molar mass silicon carbide = 40.1 g/mol
Then,
Theoretical yield = 79.1 g x (1 mol / 12.01 g) x (1 mol / 3 mol) x (40.1 g / 1 mol)
Theoretical yield = 88.04 g SiC
Therefore the theoretical yield is 88.04g
From the given values the percent yield can be computed as,
Percent yield = (actual yield of SiC / theoretical yield of SiC) x 100%
= (67.4 / 88.04) x 100%
= 0.766 x 100%
% yield of SiC = 76.6%
Therefore, the percent yield of SiC is 76.6%
To know more about percent mass, click below:
https://brainly.com/question/26150306
#SPJ1
What is the percent change when an iodine atom (I) becomes an ion (I-)?
Answers
3. KMnO4
name compound
Answers
Potassium Peramanganate .
The equation is followed as
\(\\ \ast\sf\hookrightarrow {K\atop Potassium}+{MnO_4\atop Manganeese\:Oxide}\longrightarrow {KMnO_4\atop Potassium\:Peramanganete}\)
Compare and Contrast dalton atomic. theory and Morden atomic theory.
Answers
Dalton says atoms of different factors differ in size and mass, while the cutting-edge theory says they range in common mass.
The subsequent postulates are proposed by John Dalton: All count number is manufactured from very tiny debris called atoms. Atoms are indivisible particles, which can't be created or destroyed in a chemical reaction. Atoms of a given detail are identical in mass and chemical houses.
The matter is made of indivisible debris referred to as atoms. The homes of all the atoms of a given detail are the same such as mass. this may additionally be said as all the atoms of detail have same mass at the same time as the atoms of various elements have exclusive loads.
This regulation explains that the sum of all partial pressures of gas in a combination should same as the barometric pressure. it is an especially essential calculation for oxygen to determine the pressure of gas pushing into the alveoli and consequently into the circulating blood.
Learn more about dalton atomic here:-https://brainly.com/question/13157325
#SPJ1
Three safety-related rules concerning the location of machine controls on equipment involving fluid power components.
Answers
1. Ensure Clear and Visible Placement: Machine controls should be located in a position that is easily accessible, visible, and within reach of the equipment operator. Clear and intuitive labeling or color-coding can also be used to enhance visibility and assist in identifying the controls quickly.
2. Provide Adequate Guarding: The machine controls should be positioned in a manner that minimizes the risk of accidental activation or unintended operation. This can be achieved by incorporating appropriate guarding or barriers around the controls to prevent inadvertent contact or interference.
3. Consider Ergonomics and Operator Comfort: When determining the location of machine controls, it is essential to consider ergonomic principles and operator comfort. Controls should be positioned in a way that allows operators to maintain a comfortable and natural posture while operating the equipment. This can help reduce the risk of operator fatigue, musculoskeletal disorders, and errors due to discomfort or awkward reach.
These rules aim to promote operator safety, minimize the potential for accidents, and ensure efficient and effective control of equipment involving fluid power components.
To know more about musculoskeletal disorders.
https://brainly.com/question/30279097
#SPJ11
how is the atomic radius calculated?
Answers
Explanation:
Divide the distance between the nuclei of the atoms by two if the bond is covalent
The reactants in a chemical reaction are shown below.
Li₂CO3 + H2SO4
Answers
Answer:
Li2SO4+H2CO3
Explanation:
put d equation this way
negative ions to positive ions
you prepare a 1.0 l solution containing 0.015 mol of nacl and 0.15 mol of pb(no3)2. will a precipitate form?
Answers
Since PbCl2 is insoluble, a precipitate will form when mixing 0.015 mol of NaCl and 0.15 mol of Pb(NO3)2 in a 1.0 L solution.
To determine if a precipitate will form, we need to check the solubility rules. In this case, we are interested in whether NaCl and Pb(NO3)2 will react to form any insoluble products. Here are the steps to determine that:
1. Write the balanced equation for the reaction:
NaCl (aq) + Pb(NO3)2 (aq) → NaNO3 (aq) + PbCl2 (s)
2. Identify the solubility rules:
- All nitrates (NO3-) are soluble.
- All sodium (Na+) salts are soluble.
- Chlorides (Cl-) are generally soluble, except for silver (Ag+), lead (Pb2+), and mercury (Hg2+) salts.
3. Apply the solubility rules to the products:
- NaNO3 is soluble because it contains sodium (Na+) and nitrate (NO3-).
- PbCl2 is insoluble because it is a chloride (Cl-) salt containing lead (Pb2+).
Since PbCl2 is insoluble, a precipitate will form when mixing 0.015 mol of NaCl and 0.15 mol of Pb(NO3)2 in a 1.0 L solution.
to learn more about precipitate click here:
brainly.com/question/30763500
#SPJ11