What is the hybridization of the oxygen atoms in the nitrate ion?.
Answers
The hybridization of the oxygen atoms in the nitrate ion is sp2. The hybridization of the nitrogen atom is also sp2. Nitrate ion, NO3-, has three oxygen atoms that bond with the nitrogen atom.
The fourth oxygen atom bonds with the nitrogen atom through a double bond. As a result, the oxygen atoms in nitrate ion have an sp2 hybridization.Nitrate ion has a trigonal planar shape due to the sp2 hybridization of oxygen atoms. Since the electron pairs of nitrogen and oxygen are shared, oxygen undergoes sp2 hybridization to accommodate the bonding structure. As a result, the lone pairs of oxygen in the nitrate ion are distributed in the 2p orbitals.In nitrate, nitrogen and three oxygen atoms form covalent bonds. The hybridization of the nitrogen atom in nitrate ion is also sp2 because it has three regions of electron density (one double bond and two single bonds). Hence, it is a trigonal planar molecule with bond angles of 120 degrees.150 words limitIn summary, the hybridization of the oxygen atoms in the nitrate ion is sp2, and the hybridization of the nitrogen atom is also sp2. The oxygen atoms in nitrate ion undergo sp2 hybridization to accommodate the bonding structure, and they have a trigonal planar shape. Nitrate ion is a trigonal planar molecule with bond angles of 120 degrees, and nitrogen and three oxygen atoms form covalent bonds.
To know more about nitrogen visit :
https://brainly.com/question/16711904
#SPJ11
Related Questions
Please submit as a word or pdf document. Thanks.
The links are below:
file:///C:/Users/memas/Downloads/31a5416303c7c6e221458a74e431f8ea.pdf
Answers
Answer:
no i cant Your file couldn’t be accessed It may have been moved, edited, or deleted.
Explanation:
If I add 200 mL of water to 600 mL of 16 M HNO3 what will the solution's final molarity be?
Answers
12M HNO3
Explanation:
A 600-ml solution of 16M nitric acid contains
16M HNO3 × 0.6 L = 9.6 m HNO3
so by increasing the volume by 200 ml the molarity of the solution becomes
Molarity = (9.6 mol HNO3)/(0.800 L)
= 12M HNO3
what is a flame,metion two types of flames
Answers
Answer:
A flame is defined as the gaseous part of the fire which is visible to us. A flame is created due to the high exothermic reaction.
Two types of flames based on the amount of oxygen available are:
Non-luminous or blue flame: In this type of flame the amount of oxygen is sufficiently large and it gives blue flame. For example: flame of Gas stove. Luminous flame: In this type of flame, the oxygen availability id not sufficient for the complete combustion and left unburnt carbon particles that forms yellow light flame. For example: kerosene lamp.Magnesium and Hydrochloric acid (HCl) react to form magnesium chloride (MgCl2) and hydrogen gas. If you started with 65.8 g of magnesium and 45.7 g of hydrochloric acid. What is the limiting reactant? Ensure you post calculations to prove your answer. (3 marks)
Answers
Answer:
Cl
Explanation:
n for Mg=m:Mm
=65.8:24
=2.74mol
n for Cl=m:Mm
=45.7:70
=0.65mol
According to the number of moles we can say that the limiting reagent is Cl as it has few moles.
Limiting agents are the reactant that determines the amount of the product in a reaction. In the reaction between magnesium and hydrochloric acid, the limiting reagent is HCl.
What is a limiting reagent?A limiting reagent is a substance that restricts or determines the amount of product formation in a reaction. They are the reactants with less number of moles and get consumed in the reaction before the other reactant.
The reaction between magnesium and hydrochloric acid is given by,
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H2(g)
Here, 1 mole of Mg and 2 moles of HCl are required to produce 1 mole of magnesium chloride.
The moles of Mg,
= 65.8 g ÷ 24.305
= 2.707 mol
And, moles of HCl
= 45.7 g ÷ 36.458
= 1.253 mol
The stoichiometric ratio Mg : HCl = 1:2
Hence, Mg = 2.707 ÷ 1 = 2.707 and HCl = 1.253 ÷ 2 = 0.6265
Therefore, HCl is the limiting reagent.
Learn more about limiting reagents here:
https://brainly.com/question/19654705
#SPJ5
What is the formula for Copper (1) Oxide

Answers
Answer:
Cu₂O
Explanation:
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu₂O. It is one of the principal oxides of copper, the other being or copper (II) oxide or cupric oxide. This red-coloured solid is a component of some antifouling paints.
What aspect of the experiment ensured that the number of moles in the balloon before placing it in the freezer was the same number of moles in the balloon when it was removed from the freezer
Answers
The aspect of the experiment that ensured that the number of moles in the balloon before placing it in the freezer was the same number of moles in the balloon when it was removed from the freezer is the law of conservation of matter.
What is the law of conservation of matter?The law of conservation of matter states that matter can neither be created nor destroyed but can be converted from one form to another. Thus, the number of moles in the balloon remains the same before and after it is placed in the freezer. This is the same for all experiments involving the conservation of matter.
Therefore, in the experiment, since the number of moles in the balloon is conserved, it is an assurance that the number of moles in the balloon before placing it in the freezer was the same number of moles in the balloon when it was removed from the freezer.
learn more about moles here
https://brainly.com/question/15356425
#SPJ11
Can someone help me here please i need this :(
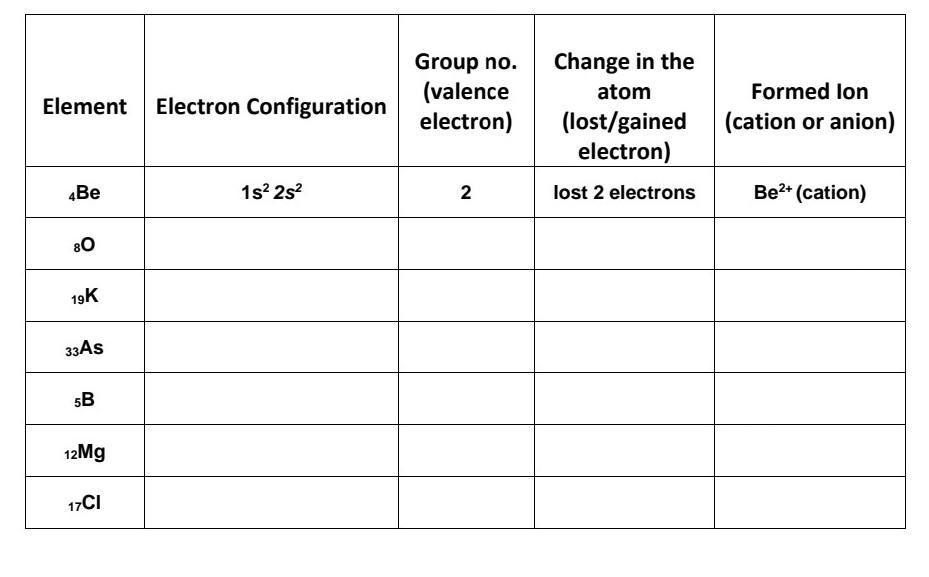
Answers
A balloon contains 3.20 L of helium. The pressure is reduced to 59.0 kPa, and the
balloon expands to occupy a volume of 5.50 L. What was the initial pressure exerted on
the balloon?
Answers
Answer:
101.4pKa
Explanation:
Given parameters:
Initial Volume of helium = 3.20L
Final pressure of helium = 59kPa
Final volume on helium gas = 5.5L
Unknown:
Initial pressure exerted on the balloon = ?
Solution;
To solve this pressure - volume problem, we simply apply the Boyle's law.
It states that "the volume of a fixed mass gas varies inversely as the pressure changes if the temperature is constant".
Mathematically;
P1 V1 = P2 V2
Now, insert parameters and solve;
P1 x 3.2 = 59 x 5.5
P1 = 101.4pKa
The water released by the reaction (mass = 0.00020 g) was calculated as was the
heat energy released (-9.6 x 10 kJ). Given the information you have about the 5
accelerants, see if you can determine which liquid is the accelerant under the
threshold.
1. Acetone:
C3H60+
02-
CO2 +
H20 ΔΗ =
2. Coleman Fuel:
C5H12 +
02-
CO2 +
H20 ΔΗ =
3. Ethyl alcohol
C2H60 +
02 -
CO2 +
H20 ΔΗ =
4. Mineral Spirits:
C10H22 +
02-
CO2 +
H20 AH =
5. Turpentine:
C10H16 +
02 -
CO2 +
H20 ΔΗ =
The accelerant used was
I
which is commonly found in:

Answers
All of the following are qualitative tests that are often used to identify unknown ionic compounds except __________
Answers
All of the following are qualitative tests that are often used to identify unknown ionic compounds except titration.
Qualitative tests are conducted to determine the presence or absence of certain ions or compounds in a sample based on their characteristic reactions. Common qualitative tests include flame tests, precipitation reactions, color changes, and gas evolution tests.
Titration, on the other hand, is a quantitative technique used to determine the concentration of a specific substance in a solution by reacting it with a standardized solution of another substance. It is not typically used as a qualitative test for identifying unknown ionic compounds.
learn more about ionic compounds here
https://brainly.com/question/30420333
#SPJ11
The physical fitness of an athlete is often measured by how much oxygen the athlete takes in (which is recorded in milliliters per kilogram, ml/kg ). The mean maximum oxygen uptake for elite athletes has been found to be 63 with a standard deviation of 7.6. Assume that the distribution is approximately normal. Find the probability that an elite athlete has a maximum oxygen uptake of at least 70.6ml/kg. Find the probability that an elite athlete has a maximum oxygen uptake of at most 52.36ml/kg. Find the probability that an elite athlete has a maximum oxygen uptake of 52.36ml/kg.
Answers
The probability that an elite athlete has a maximum oxygen uptake of at least 70.6 ml/kg is approximately 0.837.
The probability that an elite athlete has a maximum oxygen uptake of at most 52.36 ml/kg is approximately 0.0853.
The probability that an elite athlete has a maximum oxygen uptake of exactly 52.36 ml/kg is practically zero.
To find the probabilities related to the maximum oxygen uptake of elite athletes, we can use the standard normal distribution since the distribution is assumed to be approximately normal.
1 - Probability of maximum oxygen uptake of at least 70.6 ml/kg:
We need to find P(X ≥ 70.6), where X is the maximum oxygen uptake.
First, we need to standardize the value of 70.6 using the formula: z = (X - μ) / σ
where z is the standard score, X is the value, μ is the mean, and σ is the standard deviation.
z = (70.6 - 63) / 7.6
z = 0.987
Using a standard normal distribution table or calculator, we can find the probability corresponding to z = 0.987, which is approximately 0.837.
Therefore, the probability that an elite athlete has a maximum oxygen uptake of at least 70.6 ml/kg is approximately 0.837.
II - Probability of maximum oxygen uptake of at most 52.36 ml/kg:
We need to find P(X ≤ 52.36), where X is the maximum oxygen uptake.
Standardizing the value of 52.36:
z = (52.36 - 63) / 7.6
z = -1.368
Using the standard normal distribution table or calculator, we find the probability corresponding to z = -1.368, which is approximately 0.0853.
Therefore, the probability that an elite athlete has a maximum oxygen uptake of at most 52.36 ml/kg is approximately 0.0853.
III - Probability of maximum oxygen uptake exactly 52.36 ml/kg:
Since the maximum oxygen uptake is a continuous variable, the probability of obtaining an exact value is infinitesimally small. In practical terms, we can consider this probability to be effectively zero.
Therefore, the probability that an elite athlete has a maximum oxygen uptake of exactly 52.36 ml/kg is practically zero.
You can learn more about oxygen uptake at
https://brainly.com/question/33119713
#SPJ11
how much energy is produced when 93.5 grams of oxygen react with 13.2 grams of hydrogen in the following reaction:
2H2+o2-->2 h2o triangleH=-572kJ
Answers
Answer:
-1670.24 kJ
QUICK Explanation:
ΔH * moles of limiting reactant = Energy produced
-572 kJ/mol * 2.92 moles of O2 = -1670.24 kJ
LONGER EXPLANATION :
2 H2 + O2 -> 2 H2O
ΔH * moles of limiting reactant = Energy produced
enthalpy change or heat of reaction formula
1. Calculate the number of moles of oxygen (O2):
Number of moles = mass / molar mass
Number of moles of O2 = 93.5 g / 32.00 g/mol
≈ 2.92 mol O2
2. Calculate the number of moles of hydrogen (H2):
Number of moles = mass / molar mass
Number of moles of H2 = 13.2 g / 2.02 g/mol
≈ 6.53 mol H2
3. Determine the limiting reactant:
According to the balanced equation,
2 moles of H2 react with 1 mole of O2.
Calculate the moles of O2 based on the moles of H2:
(6.53 mol H2) / (2 mol H2/O2) = 3.27 mol O2
we need 3.27 mol O2 to react with the available H2
BUT only have 2.92 mol of O2 available
O2 is the limiting reactant
4.
Calculate the heat given off by assuming the complete consumption of the limiting reagent
calculate the amount of energy produced using the given enthalpy change (ΔH):
Energy produced = ΔH * moles of limiting reactant
Energy produced = ΔH * moles of O2 reacted
Calculate the energy produced using the given enthalpy change (ΔH):
Energy produced = ΔH * moles of O2 reacted
= -572 kJ/mol * 2.92 mol
≈ -1670.24 kJ
Therefore, approximately -1670.24 kJ of energy is produced when 93.5 grams of oxygen react with 13.2 grams of hydrogen in the given reaction. Note that the negative sign indicates that the reaction is exothermic (energy is released).
chatgpt
Flask is immersed in a large beaker of very hot water. At first, the level of the liquid in the tube falls, but after a short time it rises. Explain why the liquid level in the tube stops falling and starts to rise.
Answers
Answer:
Explanation:
When a flask is dipped in very hot water in a large beaker , the flask expands due to heat gain . As a result , level of water in tube fitted in flask goes down .
After some time , the water inside tube also become hot so it expands . coefficient of volume expansion of water is more than coefficient of volume expansion of glass . Hence greater expansion takes place in the volume of water . It is due to this fact that water level in tube starts rising after some time of fall .
If 2 carbon atoms, underwent fusion. What element would you get?
Answers
Answer:
Two Helium atoms
If two carbon atoms underwent fusion (of 600 million kelvin) you would get two helium atoms.
Suggest a procedure for separating iron shavings from sawdust. Explain why this procedure would work.
Dont send a file i will report you if you do
Answers
Answer:
Magnetic separation
Sawdust is a non-magnetic material whereas iron shavings are magnetic. When a magnet is brought near a mixture of iron shavings and sawdust, the iron shavings will be attracted to the magnet while the sawdust will not. Thus, the iron shavings will be separated from the sawdust.
Explanation:
Separation techniques makes use of the differences in the properties of components of mixtures to separate theses components, one from another.
In a moxturemof iron shavings and sawdust, a suitable separation technique would be the use of magnetic separation.
The use of this separation technique is abasednonnthe principle that when a magnetnis brought near to a mixture containing magnetic and non-magnetic materials, the magnetic materials will be separated from the non-magnetic materials due to their attraction to the magnet. Sawdust is a non-magnetic material whereas iron shavings are magnetic. When a magnet is brought near a mixture of iron shavings and sawdust, the iron shavings will be attracted to the magnet while the sawdust will not. Thus, the iron shavings will be separated from the sawdust.
I need this turned in soon

Answers
Answer:
Explanation:
An element's atomic number defines the amount of protons an element may contain. The atomic number is usually the big number that is shown on top. Therefore, with 17 protons it will always be chlorine.
Can you guys help me get an answer for questions (a and b) plz ‼️

Answers
Answer:
a) 15.55
b) 0.55
Explanation:
you can use geram of I2 instead of geram of NI3 in (b)
it gives the same ans

CAN SOMEONE PLEASE HELP ME OUT WITH THESE 2 QUESTIONS? ILL GIVE YOU A GOOD RATING!!

Answers
Please help me with this please!!! I’m begging you :(
Use the following terms to complete the concept map below:
malleable
brittle
metals
nonmetals
covalent bonds
electrostatic attraction

Answers
2 = nonmetals
3 = covalent bonds
4 = malleable
5 = electrostatic attraction
6 = brittle
which has the most atoms? 20g C or 70g Zn
Answers
Therefore, the 20g of C has more atoms than the 70g of Zn.
How is number of atoms determined?To determine which sample has the most atoms, we need to use Avogadro's number, which is the number of atoms in a mole. Avogadro's number is approximately 6.022 x 10^23 atoms/mole.
First, we need to determine how many moles of each substance we have. We can do this by dividing the given mass by the molar mass of the element. The molar mass of carbon (C) is approximately 12 g/mol, and the molar mass of zinc (Zn) is approximately 65 g/mol.
For 20g of C:
moles of C = 20 g / 12 g/mol ≈ 1.67 moles
For 70g of Zn:
moles of Zn = 70 g / 65 g/mol ≈ 1.08 moles
Now, to determine the number of atoms, we can multiply the number of moles by Avogadro's number.
For 20g of C:
number of atoms = 1.67 moles x 6.022 x 10^23 atoms/mole ≈ 1.00 x 10^24 atoms
For 70g of Zn:
number of atoms = 1.08 moles x 6.022 x 10^23 atoms/mole ≈ 6.50 x 10^23 atoms
Therefore, the 20g of C has more atoms than the 70g of Zn.
Learn more about moles here:
brainly.com/question/26416088
#SPJ1
If a new method for obtaining oil from dry oil fields is found, then we will see a. the AS curve shift to the left. b. a movement to the left along the AD curve. c. the AD curve shift to the left. d. the AD curve shift to the right. e. the A5 curve shift to the right.
Answers
If a new method for obtaining oil from dry oil fields is found, then we would expect to see an increase in oil production, which would lead to a leftward movement along the AD curve as demand for oil is met more easily.
However, if this increase in supply is significant enough, it could also lead to a shift of the AS curve to the right, indicating an increase in potential output. Therefore, the correct answer would be b. a movement to the left along the AD curve, and potentially a shift of the AS curve to the right. The AD curve represents the relationship between aggregate demand and the price level, while the AS curve represents the relationship between aggregate supply and the price level.
If a new method for obtaining oil from dry oil fields is found, then we will see e. the AS (Aggregate Supply) curve shift to the right. This is because the new method would increase the availability of oil, resulting in a larger supply of goods and services at the same price level, which causes the AS curve to shift to the right.
Visit here to learn more about AD curve:
brainly.com/question/29610075
#SPJ11
What chemicals are mutagens?.
Answers
A chemical or physical substance that has the ability to cause DNA mutations is referred to as a mutagen. Products like tobacco and radioactive materials are examples of mutagens.
How do we define the term "chemical"?Anything with a certain composition is considered a chemical. The same "stuff" makes up a chemical, in other words, consistently. Water, for example, contains certain compounds. Additionally, chemicals like chlorine are produced (used for bleaching fabrics or in swimming pools).
Describe some examples of chemicals.Chemicals can be formed up of simple substances like water, carbon dioxide, salt, and zinc, as well as more sophisticated ones like your computer, the atmosphere, rain, a chicken, a car, etc. Chemicals can also be made up of chemical components like oxygen, helium, and zinc.
To know more about Chemical visit:
https://brainly.com/question/29237397
#SPJ4
According to avogadros law, how will the number of molecules in 2 liters of hydrogen gas compare with the number of molecules in 2 liters of oxygen gas at the same temperature and pressure?
A hydrogen will have more molecules in a volume of 2 liters
B oxygen will have more molecules in a volume of 2 liters
C they will have equal number of molecules
D there is no way to tell
Answers
Answer:
C - they will have equal number of molecules.
Explanation:
The number of molecules in 2 liters of hydrogen gas compare with the number of molecules in 2 liters of oxygen gas at the same temperature and pressure because while the equal volumes of the gases are at the same temperature and pressure, it still has the same number of molecules.
Suppose you were given a substance and asked
to determine whether or not it was a plasma. Write
the characteristics you would look for to identify
the substance.
Answers
Answer:Particles in plasmas collide more often.
Plasma particles have high kinetic energy (they move quickly).
Plasma particles are far apart.
The ionized particles have no fixed volume
Explanation:
Plasma is the fourth form of matter which has freely moving electrons in it. The substance can be identified as plasma if its particles collide often and are far apart.
What is plasma?Plasma is a type of matter other than solids, liquids, and gas. They have similar properties to that of gas. Plasma particle collides are often like gases as they move freely in space. Like gases that have no definite shape and volume.
The plasma particles have ionic charges, positive and negative resulting in high kinetic energy. This property allows them to show electromagnetism and electrical conductivity.
The ionized particles are due to the high temperature that allows them to have the property of electrical conductivity and compression.
Therefore, the particles of plasma are ionized and have high kinetic energy.
Learn more about plasma here:
https://brainly.com/question/10133920
#SPJ6
If the atomic number of an element is 6 and the atomic mass is 12.01, how many protons are there in the nucleus?
A. 12
B. 6
C. 24
D. 52
Answers
The atomic number of an element represents the number of protons in the nucleus. In this case, the atomic number is 6. Therefore, there are 6 protons in the nucleus of this element. The correct answer is B. 6.
The atomic number of an element represents the number of protons in the nucleus. In this case, the atomic number is 6, which means there are 6 protons in the nucleus. The atomic mass is the sum of the number of protons and neutrons in the nucleus. Since the atomic mass is 12.01 and the atomic number is 6, we can subtract 6 from 12.01 to get the number of neutrons. This gives us a neutron count of approximately 6.01.
Therefore, The answer is B. 6 protons are in the nucleus of this element.
Learn More about nucleus here :-
https://brainly.com/question/21083478
#SPJ11
An aqueous solution of non-electrolyte A, with a molecular
mass of 60, contains 6 grams of non-electrolyte A in 500 mL
and has a density equal to 1.05 g per mL. The molality of the
solution is
Answers
Answer:0.19m
Explanation:
Molality (m) of the solution is = 0.192 mol/kg. Molality (also molal concentration) is represented by symbol m, it measures the number of moles of solute (here a non-electrolyte solute A) present in 1 kg of solvent. Here, moles of solute can be calculated by dividing given mass of non-electrolyte A with molecular weight of A.
Molecular mass of non-electrolyte A = 60g
Mass of non-electrolyte A = 6g
Volume of solution = 500mL
Density of solution = 1.05g
Calculating molality of solutionTo calculate molality of solution using formula of molality:
Molality = moles of solute / kilograms of solvent (mol/kg). Here we need to calculate mass of the solvent in kg that is not directly given in the question. Hence, it is required to calculate mass of the solution first and then subtract the mass of solute from it. Also, to calculate the mass of solution, density and volume are given. So, the calculations are done as follows;
Firstly,
To calculate mass of solution using formula of density; D (ρ) = mass/volume. Density (ρ) is the mass of substance divide by it's volume. Therefore, Mass of solution = Density × volume
Mass of solution = 1.05 × 500
Mass of solution = 525 g
Now, Mass of solvent is calculated as Mass of solute (non-electrolyte A) subtracted from mass of solution. Therefore,
Mass of solvent = 525 - 6 = 519 g (0.519 kg)
Moles of solute are calculated as given weight of solute divided by molecular weight of solute. Hence, moles of solute = 6/60
Moles of solute = 0.1
Molality of solution is calculated as moles of solute divided by mass of solvent or kilograms of solvent (mol/kg)
Molality of solution = 0.1/ 0.519
Molality (m) = 0.192 mol/kg
Learn more about molality here
https://brainly.com/question/20018472
#SPJ2
calculate the entropy difference (δs) between the two systems a and b. express your answer in the correct units and to the correct number of significant figures
Answers
Systems A and B, such as their temperatures, volumes, or any other relevant details, it is not possible to calculate the entropy difference accurately. Entropy is a state function that depends on the specific conditions of a system.
To calculate the entropy difference between two systems, we typically compare the entropy of the initial state (A) to the entropy of the final state (B). This requires knowledge of the specific properties and conditions of both systems.
Entropy is commonly expressed in units of joules per Kelvin (J/K). The entropy difference, ΔS, is calculated as the difference between the entropy of the final state (Sf) and the entropy of the initial state (Si): ΔS = Sf - Si.
If you can provide additional details about systems A and B, such as their temperatures, volumes, or any other relevant parameters.
Learn more about Entropy here:
https://brainly.com/question/20166134
#SPJ11
how many ml of a 4% solution must be added to 500 ml of iv solution to achieve a solution concentration of 4 mg/ml?
Answers
Hence we have to add 20 ml of 4% solute in order to obtain a solution concentration of 4 mg/ml.
4 percent solution means 4 parts of solute are dissolved in 100 parts of solvent.
Therefore 4mg / 100ml = x mg / 500 ml
X= 4 * 500 / 100
X = 20
Concentration is a totally commonplace idea utilized in chemistry and associated fields. It's far the degree of the way an awful lot of a given substance there may be combined with some other substance.
The concentration of an answer is the degree of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a fantastically large amount of dissolved solute.
In chemistry, concentration refers to the quantity of a substance in a defined space. Any other definition is that attention is the ratio of solute in a solution to either solvent or overall answer.
Learn more about Concentration here:-brainly.com/question/26255204
SPJ4
name the structure in the figure in which an electron transport chain is located. describe the main function of the processes that occur in this structure.
Answers
The structure in the figure where an electron transport chain is located is the inner mitochondrial membrane.
The main function of the processes that occur in the inner mitochondrial membrane, specifically in the electron transport chain, is to generate ATP through oxidative phosphorylation.
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. It plays a crucial role in the final stage of cellular respiration, which is the process by which cells extract energy from nutrients.
During oxidative phosphorylation, electrons are transferred through the electron transport chain from energy-rich molecules such as NADH and FADH2.
As electrons pass through the protein complexes, their energy is gradually released, and protons (H+) are pumped across the inner mitochondrial membrane from the mitochondrial matrix to the intermembrane space. This creates an electrochemical gradient.
The main function of this electron transport and proton pumping is to establish a proton motive force.
The gradient created by the electron transport chain drives the ATP synthase enzyme, located in the inner mitochondrial membrane, to produce ATP from ADP and inorganic phosphate. This process is known as chemiosmosis.
Overall, the electron transport chain in the inner mitochondrial membrane plays a crucial role in generating ATP, the energy currency of the cell, by utilizing the energy stored in the electrons derived from the breakdown of nutrients.
To know more about "Mitochondrial membrane" refer here:
https://brainly.com/question/31797295#
#SPJ11
Are elements matter and why?
Answers
All matter is made up of substances called elements, which have specific chemical and physical properties and cannot be broken down into other substances through ordinary chemical reactions. Gold, for instance, is an element, and so is carbon. There are 118 elements, but only 92 occur naturally.
PLEASE MARK ME BRAINLIEST.