Answers
Answer:
C
Explanation:
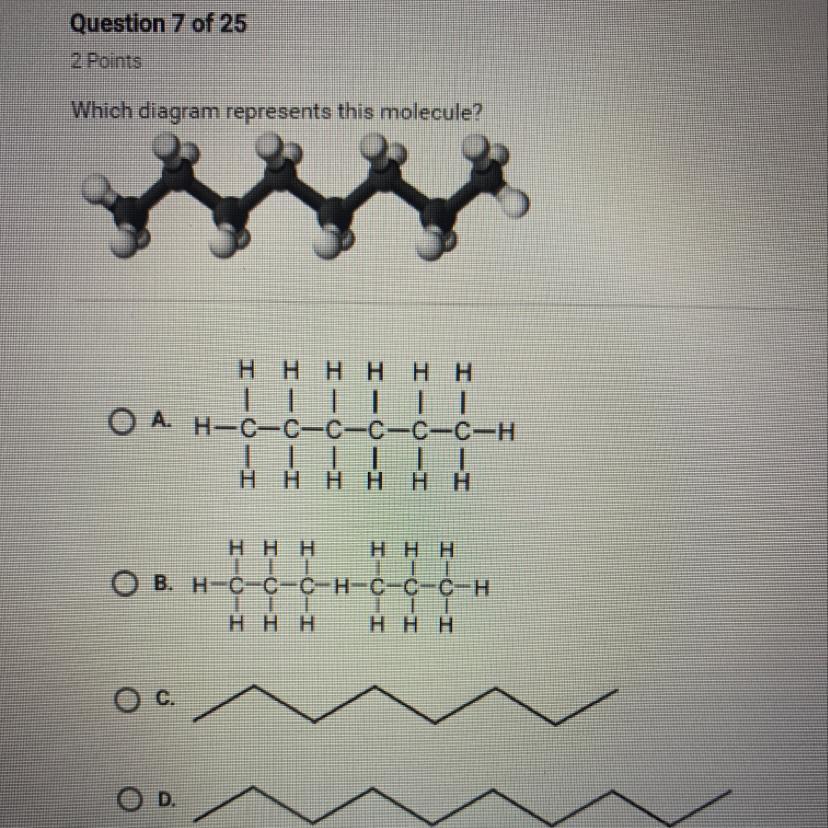
The molecule has 8 carbon atoms joined by 7 C-C bonds.
The first two diagrams show 6 carbon atoms, not 8.
The last two diagrams show line segments representing C-C bonds. Only choice C shows 7 such segments.
The appropriate choice is C.
Answer:
C.
Explanation:
Related Questions
A gas has a pressure of 2.70 atm at 50.0 °C. What is the pressure at standard temperature (0°C)?
Answers
Answer:
2.282 atm
P1V1/T1 = P2V2/T2
2.70atm / (50+273) = X/ 273
make x subject of formula
:. X = 2.28 atm
or 2.28 * 1.01 *10⁵ N/m²
you can support by rating brainly it's very much appreciated ✅✅
It took 412.5 J of heat to raise the temperature of 24.6 g of a substance from 14.5°C to 52.6°C. What is the specific heat of the substance?
Answers
If it took 412.5 J of heat to raise the temperature of 24.6 g of a substance from 14.5°C to 52.6°C, the specific heat of the substance is 0.44 J/g°C.
How to calculate specific heat of a substance?The specific heat of a substance can be calculated by using the following formula:
Q = m × c × ∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substancec = specific heat capacity∆T = change in temperature412.5 = 24.6 × c × (52.6 - 14.5)
412.5 = 937.26c
c = 412.5/937.26
c = 0.44 J/g°C
Therefore, If it took 412.5 J of heat to raise the temperature of 24.6 g of a substance from 14.5°C to 52.6°C, the specific heat of the substance is 0.44 J/g°C.
Learn more about specific heat at: https://brainly.com/question/1747943
#SPJ1
Polyethylene is 86.0% C and 14.0%
H. Determine the empirical formula of the compound.
Percent to Mass: How many grams of C/and Hare present in 100.0 g?
Answers
The empirical formula of polyethylene can be determined by converting the given percentages of carbon (C) and hydrogen (H) into grams. To find the grams of each element, we assume a 100.0 g sample of polyethylene.
For carbon:
Mass of carbon = 86.0% × 100.0 g = 86.0 g
For hydrogen:
Mass of hydrogen = 14.0% × 100.0 g = 14.0 g
Therefore, in a 100.0 g sample of polyethylene, there are 86.0 grams of carbon and 14.0 grams of hydrogen.
The empirical formula of a compound represents the simplest whole-number ratio of atoms present in the compound. To determine the empirical formula, we need to find the ratio of carbon to hydrogen in terms of moles.
First, we convert the masses of carbon and hydrogen into moles using their respective molar masses. The molar mass of carbon is approximately 12.01 g/mol, and the molar mass of hydrogen is approximately 1.008 g/mol.
Number of moles of carbon = 86.0 g / 12.01 g/mol ≈ 7.162 mol
Number of moles of hydrogen = 14.0 g / 1.008 g/mol ≈ 13.89 mol
Next, we divide the number of moles of each element by the smallest number of moles to get a simplified ratio.
Carbon: Hydrogen ≈ 7.162 mol : 13.89 mol ≈ 1 : 1.939
Since we want to express the ratio in whole numbers, we multiply both sides by 2 to get a whole number ratio.
Carbon: Hydrogen ≈ 2 : 3.878
Rounding to the nearest whole number, we find that the empirical formula of polyethylene is CH₂.
for such more questions on hydrogen
https://brainly.com/question/24433860
#SPJ8
label the sun. which layer of the sun is #6?

Answers
Answer: Corona
Explanation: hope this help
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
help plssss!!!! i need this done by tonight!!!!

Answers
1. Using your knowledge of the Brønsted-Lowry theory of acids and bases, complete the following acid-base reactions and indicate each conjugate acid-base pair.
i. OH + HPO₂ → H₂O + H₂PO₄²⁻
The conjugate acid-base pair is OH/H₂O, HPO₂²⁻/H₂PO₄²⁻
2) Identify the conjugate acid-base pairs in the following reactions. Write A, B, CA, and CB below the appropriate substance.
i. HCO₃⁻ + NH₃ → NH₄⁺ + CO₃²⁻
The conjugate acid-base pair is HCO₃⁻/CO₃²⁻, NH₃/NH₄⁺
ii. HCI + H₂O → H₃O⁺ + Cl⁻
The conjugate acid-base pair is H₂O/OH⁻, HCI/Cl⁻, H₃O⁺/H₂O, Cl⁻/HCI
iii. CH₃COOH + H₂O → H₃O⁺ + CH₃COO⁻
The conjugate acid-base pair is CH₃COOH/CH₃COO⁻, H₂O/OH⁻, H₃O⁺/H₂O
iv. HOCI + NH₃ → NH₄⁺ + ClO⁻
The conjugate acid-base pair is HOCI/ClO⁻, NH₃/NH₄⁺
3. Write the formula for conjugate bases formed by the following acids.
i. HPO₄²⁻ → PO₄³⁻
ii. H₂O → OH⁻
iii. CN⁻ → HCN
iv. HOOC-COO⁻ → HOOCCOOH
4) Write the formula for conjugate acids formed by each of the following bases.
i. H₃O⁺ → H₂O
ii. HCN → H₂CN⁺
iii. NH₃ → NH₄⁺
5. Classify each of the following pH values as acidic, basic, or neutral.
10 - neutral1.5 - acidic7 - neutral7.5 - basic13 - basic1 - acidicLearn more about Conjugate base pairs here:
https://brainly.com/question/13336099
#SPJ1
Calculate ΔHrxn for the following reaction: Al2O3(s)+3CO(g)→2Al(s)+3CO2(g) Use the following reactions and given ΔH values: 2Al(s)+32O2(g)→Al2O3(s),ΔH CO(g)+12O2(g)→CO2(g),ΔH==−1675.7kJ−282.7kJ
Answers
The desired reaction is 2Al(s) + 3CO2 from Al2O3(s) + 3CO(g) (g) The reactions include 2 Al(s), 3/2 O2(g), and Al2O3(s), with H = 1675.7kJ. ————————— (1) CO(g) = CO2 + 1/2 O2(g) (g).
How is H inside a calculated?As a result, the enthalpies of a reactants and products are added together, and the result is used to compute the enthalpy of a reaction. This endothermic process generates and absorbs environmental heat if H is positive. This reaction is exothermic so emits heat into the environment if H is negative.
What is the H heat?A negative H indicates that heat is transferred from the a system towards its surroundings, whereas a positive H indicates that heat is transferred from the surroundings into the system. An enthalpy of reaction (Hrxn) for a chemical reaction is the difference of enthalpy between the products and reactants; Hrxn is measured in kilojoules per mole.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
You are standing in the yard with your big dog on a leash when another dog walks down your street.
Your dog wants to get to the other dog so he starts to pull on his leash with a force of 25 N. You can
only pull back with a force of 25 N. What will happen to you?
The forces are unbalanced so the dog will pull you forward.
The forces are balanced so you and the dog will not move.
The forces are unbalanced so the you will pull the dog backward.
Answers
Answer:
C
Explanation:
it should be C
suppose you have a sample of two pure substances: a solid piece of ice and a solid piece of copper metal. Which would be easier to break apart? Explain your reasoning
Answers
Answer:
Solid piece of ice.
Explanation:
Solid piece of ice can easily melt due to heat radiation
Question 6 (1 point)
"When two or more objects collide, there will be the same amount of momentum before the collision as after" is
a) Momentum Rule
b) Law of Collisions
c) Law of Conservation of Momentum
d) The Law of Before and After
Answers
Describing How to Convert Between Moles, Liters, and
Mass
When hot lava reaches seawater, the salts in the
water react with steam to form gaseous
hydrochloric acid. You are given an unbalanced
chemical equation for one such reaction and the
volume of HCI(g) produced. To the right are the
steps to explain how you would find the mass of
solid sea salt needed to produce the given gas
volume. Choose the correct order of the steps by
selecting the correct step number in the drop-
down.
Balance the chemical equation.
Multiply by the molar mass of the salt and convert
moles to mass.
Use the balanced equation to find out how many
moles of the salt are needed to produce the moles
of HCI.
Convert the volume of HCI to mol HCI by dividing
by the molar volume.
DONE
Answers
Moles can be converted to liters by the use of the stoichiometry of the reaction and the gas laws.
How can you convert moles to liters?To convert moles to liters, you need to use the Ideal Gas Law. The Ideal Gas Law states that the number of moles of a gas is proportional to its volume at a given temperature and pressure. The equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
By rearranging the equation, you can find the volume of a gas in liters when the number of moles is known:
V = nRT / P
Since the volume is in liters, the number of moles can be converted to liters by multiplying it by the volume.
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
A photon of light hits the
electron in the image. What-
happens to the electron,
assuming there is enough
energy?
Answers
Answer:
When a photon of light hits an electron, the photon transfers some of its energy to the electron. If the photon has enough energy, it can excite the electron, which means that it can raise the electron to a higher energy state. In other words, the photon's energy can be absorbed by the electron, causing the electron to move to a higher energy level within an atom or molecule.
Answer:
Explanation:
As we know that in the photoelectric effect, a low-energy gamma photon that collides with an atom can tranfer all its energy to an inner orbital electron and cause the ejection of the ELECTRON FROM THE ATOM
The amount of energy required to eject the electrons depends upon on the atomic number.
you design an experiment to test the effect of adding different amounts of ice (grams) to a given volume of water.
For each trial you record the initial temperature of the water and then the final temperature after the ice was added.
In this experiment, the amount of ice is the
variable, and the temperature change is the
variable.
Answers
Answer:
In this experiment, the amount of ice is the independent variable, and the temperature change is the dependent variable.
For the reaction
2NH3(g)↽−−⇀ 3H2(g)+N2(g)
the equilibrium concentrations were found to be [NH3]=0.250 M, [H2]=0.470 M, and [N2]=0.800 M. What is the equilibrium constant for this reaction?
eq=
Answers
Rounding to the appropriate number of significant figures, the equilibrium constant (Kc) for the reaction is approximately 1.66.
To calculate the equilibrium constant (Kc) for the given reaction, we can use the formula:
Kc = ([H2]^3 * [N2]) / [NH3]^2
Plugging in the given equilibrium concentrations, we have:
Kc = (0.470^3 * 0.800) / (0.250^2)
Calculating the numerator:
(0.470^3 * 0.800) = 0.1037032
Calculating the denominator:
(0.250^2) = 0.0625
Now, dividing the numerator by the denominator:
Kc = 0.1037032 / 0.0625 = 1.6592512
The equilibrium constant represents the ratio of the concentrations of products to reactants at equilibrium. In this case, the equilibrium constant is greater than 1, indicating that the products (H2 and N2) are favored at equilibrium. This means that the forward reaction is favored, leading to the formation of more products compared to reactants.The equilibrium constant value of 1.66 suggests that the forward reaction is moderately favored at equilibrium, but without additional context, it is difficult to determine the extent of the reaction or the relative concentrations of reactants and products at the beginning of the reaction.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
2 C4H10 + 13O2 -------> 8CO2 + 10H2O
a) What is the mole ratio between butane and oxygen gas?
b) What is the mole ratio between water and oxygen gas?
c) How many moles of water formed?
d) How many moles of butane burned?
e) How many grams of butane burned?
f) How much oxygen was used up in moles?
g) How much oxygen was used up in grams?
Answers
Answer:
A. 2 : 13
B. 10 : 13
C. 10 moles of water, H₂O.
D. 2 moles of butane, C₄H₁₀
E. 116 g of butane, C₄H₁₀
F. 13 moles of oxygen, O₂
G. 416 g of oxygen, O₂
Explanation:
The equation for the reaction is given below:
2C₄H₁₀ + 13O₂ —> 8CO₂ + 10H₂O
A. Determination of the mole ratio between butane and oxygen gas.
Mole of butane, C₄H₁₀ = 2 moles
Mole of oxygen, O₂ = 13 moles
Mole ratio of butane and oxygen = 2 : 13
B. Determination of the mole ratio between water and oxygen gas
Mole of the water, H₂O = 10 moles
Mole of oxygen, O₂ = 13 moles
Mole ratio of water and oxygen = 10 : 13
C. Determination of the moles of water formed.
From the balanced equation above,
10 moles water, H₂O were produced.
D. Determination of the moles of butane burned.
From the balanced equation above,
2 moles of butane, C₄H₁₀ were burned.
E. Determination of the mass of butane burned.
Molar mass of C₄H₁₀ = (12×4) + (10×1)
= 48 + 10 = 58 g/mol
Mole of C₄H₁₀ = 2 moles
Mass of C₄H₁₀ =?
Mass = mole × molar mass
Mass of C₄H₁₀ = 2 × 58
Mass of C₄H₁₀ = 116 g
Thus, 116 g of butane, C₄H₁₀ were burned.
F. Determination of the number of mole of oxygen used.
From the balanced equation above,
13 moles of oxygen, O₂ were used.
G. Determination of the mass of oxygen used.
Molar mass of O₂ = 2 × 16 = 32 g/mol
Mole of O₂ = 13 moles
Mass of O₂ =?
Mass = mole × molar mass
Mass of O₂ = 13 × 32
Mass of O₂ = 416 g
Thus, 416 g of oxygen, O₂ were used.
_ S8 +_02 +_ SO3
How many grams of sulfur are needed to produce 167.82 grams of sulfur trioxide?
6.547 g sulfur
4302 g sulfur
67.22 g sulfur
537.7 g sulfur
Answers
if the barometric reading is at 28.09 in what kind of weather do you predict they may have?
Answers
Answer:
In general, a falling barometer indicates the approach of a storm. If the mercury is over 30.20 inches but falling quickly, warmer, cloudier weather is coming. If the mercury continues to fall, the weather will worsen. When the mercury level is between 30.20 and 29.80 inches and dropping rapidly, expect precipitation.
Explanation:
Answer: idek warm?
Explanation:
A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she tried the following experiment. First she measured the mass of the metal to be 385.8 grams. Then she dropped the metal into a measuring cup and found that it displaced 17.8 mL of water. Calculate the density of the metal. Density = _______ g/mL Use the table below to decide the identity of the metal. This metal is most likely _________.
substances density g/cm3
water 1.00
aluminium 2.72
chromium 7.25
nickel 8.91
silver 10.50
lead 11.34
Answers
1. 21.67g/ml
2. aluminium
Explanation:
1. density = mass/volume
385.8/17.8= 21.67ml
2. 1g/ml=0.1g/cm^3
21.67g/ml = 2.167g/cm^3
..... substance is probably aluminium
1. the density of the metal is 21.67g/ml
2. This metal is most likely aluminum
The calculation is as follows;
1.
\(density = mass \div volume\)
\(385.8\div 17.8= 21.67ml\)
2.
1g/ml=0.1g/cm^3
So,
21.67g/ml = 2.167g/cm^3
Therefore, substance is probably aluminum
Learn more: https://brainly.com/question/17429689?referrer=searchResults
The state of matter of a substance is a physical property. A. True B. False
Answers
Answer:
I THINK IT IS TRUE BECAUSE THEY ERE FIRST FOUND TO DEMOCRITUS ARISTOTLE AND PLATO THE ROMANS AND GREEK CHEMISTS .
Explanation:
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
A chemist needs to know the mass of a sample of to significant digits. He puts the sample on a digital scale. This is what the scale shows:
0
0
0
7
6
.
3
g
If this measurement is precise enough for the chemist, round it to significant digits.
Otherwise, press the "No solution" button.
Answers
In type of scientific measurement, the precision of the measurement is expressed in the significant digits of that measurement. It also used to express measurement to the required degree of accuracy.
Significant digits include every digit except the leading zero(s).
And if the number after the required significant digits is not up to 5, it is rounded down and the required significant digits is written as is. If the number after the required significant digits is at least 5, it is rounded up, and the last number on the significant digits requirement is increased by a factor of 1.
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
2 A high school student takes a lump of magnesium with a volume of 150.0 mL and adds it to a beaker of
an aqueous solution of aluminum nitrate. What is the mass of the solid aluminum that forms?
Solid magnesium has a density of 1.738 g/cm³.
Answers
The mass of the solid aluminum that forms are 192.73 grams
To determine the mass of solid aluminum that forms, we need to use stoichiometry and the balanced chemical equation for the reaction between magnesium and aluminum nitrate.
The balanced chemical equation is:
3 Mg + 2 Al(\(NO_{3}\))3 → 3 Mg(\(NO_{3}\))2 + 2 Al
The equation shows that 3 moles of magnesium react with 2 moles of aluminum to produce 2 moles of aluminum nitrate.
To calculate the mass of solid aluminum, we need to know the amount of magnesium used. Given that the volume of the magnesium is 150.0 mL and its density is 1.738 g/cm³, we can calculate the mass of magnesium using the formula:
Mass = Volume × Density
Mass of magnesium = 150.0 mL × 1.738 g/cm³ = 260.7 g
Now, using the molar mass of magnesium (24.31 g/mol) and the molar ratio from the balanced equation, we can determine the moles of magnesium used:
Moles of magnesium = Mass of magnesium / Molar mass of magnesium
= 260.7 g / 24.31 g/mol
= 10.72 mol
According to the stoichiometry of the balanced equation, the ratio of moles of magnesium to moles of aluminum is 3:2. Therefore, the moles of aluminum formed will be:
Moles of aluminum = (2/3) × Moles of magnesium
= (2/3) × 10.72 mol
= 7.15 mol
Finally, we can calculate the mass of solid aluminum using its molar mass (26.98 g/mol):
Mass of aluminum = Moles of aluminum × Molar mass of aluminum
= 7.15 mol × 26.98 g/mol
= 192.73 g
Therefore, the mass of the solid aluminum that forms is approximately 192.73 grams.
Know more about the Balanced chemical equation here:
https://brainly.com/question/13451900
#SPJ8
The helium tank has a pressure of 650 torr at 25 degree celsius what will be the pressure if the temperature is tripled?
pa help po
Answers
The helium tank has a pressure of 650 torr at 25 degree Celsius and when the temperature is tripled, the pressure will be approximately 1945.71 torr
To find the new pressure when the temperature is tripled, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and the number of particles remain constant. The ideal gas law is given by the equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 degrees Celsius to Kelvin. Adding 273.15 to the Celsius temperature gives us 298.15 K.
Let's assume that the volume, number of moles, and the gas constant remain constant.
If the temperature is tripled, the new temperature would be 3 times the initial temperature, which is 3 * 298.15 K = 894.45 K.
Now, we can set up a proportion to find the new pressure:
P1 / T1 = P2 / T2
Solving for P2 (the new pressure), we get:
P2 = (P1 * T2) / T1
Plugging in the values, we have:
P2 = (650 torr * 894.45 K) / 298.15 K
Calculating this expression, we find:
P2 ≈ 1945.71 torr
Therefore, when the temperature is tripled, the pressure will be approximately 1945.71 torr.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
Why do we feel cold when we touch the iron?
A) Heat flows from iron to our body.
B) Heat flows from our body to iron.
C) Heat flows from iron to iron
D) Heat does not not flow
Please answer it soon as possible
Answers
Heat flows from our body to iron
HCl(50ml) + NaOH(50ml) --> NaCl+H2O
Calculate the value of heat released (Q = mcT) and the Delta H
Time | Temperature
0s 22C
10s 27C
20s 29C
30s 30C
Answers
Answer:
To calculate the heat released in this reaction, we need to use the formula:
Q = mcΔT
where Q is the heat released, m is the mass of the solution, c is the specific heat capacity of the solution, and ΔT is the change in temperature.
Assuming the density of the solution is 1 g/mL, the mass of the solution is 100 g (50 mL HCl + 50 mL NaOH). The specific heat capacity of the solution can be assumed to be the same as that of water, which is 4.18 J/g°C.
The change in temperature can be calculated as the final temperature minus the initial temperature:
ΔT = 30°C - 22°C = 8°C
Therefore, we have:
Q = (100 g) * (4.18 J/g°C) * (8°C) = 3344 J
The heat released in the reaction is 3344 J.
The value of ΔH for the reaction can be calculated using the formula:
ΔH = -Q/n
where Q is the heat released, and n is the number of moles of limiting reactant used in the reaction. In this case, the limiting reactant is NaOH, and we can calculate the number of moles of NaOH from its concentration and volume:
n(NaOH) = (0.1 L) * (1 mol/L) = 0.1 mol
Therefore, we have:
ΔH = -(3344 J) / (0.1 mol) = -33,440 J/mol
The value of ΔH for the reaction is -33,440 J/mol, which is negative because the reaction is exothermic (heat is released).
4. Calculate the number of moles in each of the following quantities:
a. 6.022 X 1024 atoms of cobalt
Answers
There would be 10 moles of cobalt in 6.022 x \(10^{24\) atoms of cobalt.
Avogadro's numberIn order to calculate the number of moles in a given number of particles, we need to divide the number of particles by Avogadro's number.
6.022 x \(10^{24\) atoms of cobalt:
Number of moles = (number of particles) / Avogadro's numberNumber of moles = 6.022 x 10^24 / 6.022 x 10^23Number of moles = 10Therefore, there are 10 moles of cobalt in 6.022 x \(10^{24\) atoms of cobalt.
More on Avogadro's number can be found here: https://brainly.com/question/11907018
#SPJ1
what is kinetic energy of a 155 kg object moving at speed of 20 m/s
Answers
Answer:
KE = 1/2*m*v^2
KE = 1/2*150kg*(20 m/s)^2
KE = 75kg * 400m²/s²
KE = 30,000 kg*m²/s²
KE = 30,000 N*m
KE = 30,000 J
Explanation:
Hope this helped.
A brainliest is always appreciated.
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium? CO(g) + Cl2(8)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of Cl2(g) present is approximately 347.37 mol.
To determine the number of moles of Cl2(g) at equilibrium, we need to use the given equilibrium constant (Kc) and set up an ICE table to track the changes in the reactants and products.
The balanced equation for the reaction is:
CO(g) + Cl2(g) ⇌ COCl2(g)
Let's set up the ICE table:
CO(g) + Cl2(g) ⇌ COCl2(g)
Initial: 0.3500 0.05500 0
Change: -x -x +x
Equilibrium: 0.3500 - x 0.05500 - x x
Using the equilibrium concentrations in the ICE table, we can write the expression for the equilibrium constant (Kc) as:
Kc = [COCl2(g)] / [CO(g)][Cl2(g)]
Substituting the values into the equation, we have:
1.2 × 10^3 = (0.05500 - x) / [(0.3500 - x)(0.05500 - x)]
Simplifying the equation, we can cross-multiply and rearrange:
1.2 × 10^3 × (0.3500 - x)(0.05500 - x) = 0.05500 - x
Expanding and rearranging, we get:
0 = (1.2 × 10^3 × 0.05500 - 1.2 × 10^3x + 0.05500x) - x
Simplifying further:
0 = 66 - 1.245x + 0.05500x - x
0 = 66 - 0.19x
0.19x = 66
x = 66 / 0.19
x ≈ 347.37
For more such questions on equilibrium visit:
https://brainly.com/question/19340344
#SPJ8
What might happen if water molecules did not have a slight negative charge on one end and a slight positive charge on another
Answers
Water molecules did not have a slight negative charge on one end and a slight positive charge on another, the loss of polarity would have profound effects on various biological, chemical, and physical processes. The unique properties of water that are vital for life as we know it would be significantly altered, potentially rendering many biological systems nonfunctional and disrupting the stability of ecosystems.
Loss of hydrogen bonding: The polarity of water molecules allows them to form hydrogen bonds with each other and with other polar substances.Hydrogen bonds are relatively weak but essential for various biological processes, including protein folding, DNA structure, and the stabilization of cell membranes. Altered solubility: Water's polarity contributes to its excellent solvent properties. It can dissolve a wide range of substances, including salts, sugars, and polar molecules, due to its ability to surround and separate charged or polar particles. Changes in boiling and freezing points: The polarity of water affects its boiling and freezing points. Water has a relatively high boiling point and melting point compared to other substances of similar molecular weight. Altered surface tension: Surface tension is the cohesive force that holds the surface of a liquid together. Water exhibits relatively high surface tension due to the cohesive forces between water molecules resulting from their polarity. Changes in heat capacity: Water's ability to absorb and retain heat is crucial for temperature regulation in many organisms and helps moderate temperature changes in the environment.For such more question on Water molecules
https://brainly.com/question/21426318
#SPJ8