Answers
Answer:
C. Diminish.
Explanation:
An antonym is the opposite meaning of a given word. It is the word that gives the complete opposite meaning of the given word.
The word "enhance" means "to increase, intensify, magnify, to make it more than it already is" etc. So, the antonym of that word will be "to diminish, to reduce, to prevent" etc.
Thus, the correct answer is option C.
Related Questions
ws oxidation number and balancing equation
Answers
Oxidation number is a number assigned to an atom in a chemical compound that represents the number of electrons lost or gained by the atom.
What is balancing equation?A balancing equation or balanced is a chemical expression wherein the tally of atoms for each constituent element mirrors harmoniously on either side of the equation. This harmonious alignment extends to both mass and charge, embodying equilibrium throughout.
The art of balancing equations holds great significance as it upholds the revered principles of the conservation of mass and the conservation of charge.
The conservation of mass, a fundamental decree, dictates that the essence of matter remains unaltered, impervious to creation or annihilation in the course of a chemical transformation.
Learn about oxidation number here https://brainly.com/question/15167411
#SPJ1
Complete question:
What is oxidation number and balancing equation?
Can anyone help me understand how to calculate the moles of H+ and OH-?

Answers
To calculate the moles of H+ and OH-, you need to know the concentration of the solution in terms of its pH or pOH value.
How to calculate the molesWhen you get the pH of the solution, you can use this formula to calculate the concentration of H+ ions: [H+] = 10^(-pH)
Also, if you know the pOH of the solution, you can use this formula to calculate the concentration of OH- ions: [OH-] = 10^(-pOH)
Having determined the concentration of H+ and OH- ions, the molarity formula can be used to calculate the number of moles of each ion as follows: moles = concentration (in mol/L) x volume (in L)
Learn more about moles calculation here:
https://brainly.com/question/14357742
#SPJ1
HELP PLSSS
Why is the water cycle important for ecosystems?
A. The water cycle helps plants keep and store water. The cycle makes sure that water stays at earth's surface and is available to ecosystems.
B. The water cycle photosynthesis to make clouds. These clouds make surface water that animals drink to survive in there ecosystem.
C. The water cycle permanently removes water from the underground and from large bodies of water and transports it to terrestrial ecosystems
D. The water cycle helps recycle and transport water around the planet. The cycle replenishes the water supply of ecosystems.
Answers
Answer:
The water cycle is often taught as a simple circular cycle of evaporation, condensation, and precipitation. Although this can be a useful ...
Explanation:
Answer: Your answer will be D)
Explanation: Hope this helped
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
How many formula units are in 50.0g of Pb02?
Answers
There are approximately \(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
To solve this problem
We must utilize the molar mass of PbO2 (lead dioxide) and the idea of Avogadro's number to calculate the number of formula units in a given mass of PbO2.
The molar mass of PbO2 is calculated as follows:
1 atom of Pb (lead) has a molar mass of approximately 207.2 g/mol.
2 atoms of O (oxygen) have a combined molar mass of approximately 32.0 g/mol (16.0 g/mol per oxygen atom).
Therefore, the molar mass of PbO2 is:
Molar mass of PbO2 = (1 * molar mass of Pb) + (2 * molar mass of O)
= (1 * 207.2 g/mol) + (2 * 16.0 g/mol)
= 207.2 g/mol + 32.0 g/mol
= 239.2 g/mol
Now, we can use the molar mass to determine the number of formula units in 50.0 g of PbO2.
Number of moles = Mass (in grams) / Molar mass
= 50.0 g / 239.2 g/mol
≈ 0.209 moles (rounded to three decimal places)
Since 1 mole of any substance contains Avogadro's number of particles \((approximately 6.022 x 10^2^3),\)we can calculate the number of formula units by multiplying the number of moles by Avogadro's number:
Number of formula units = Number of moles * Avogadro's number
\(= 0.209 moles * (6.022 x 10^2^3 formula units/mole)\)
≈\(1.258 x 10^2^3 formula units\)
Therefore, there are approximately\(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
Learn more about molar mass here : brainly.com/question/21334167
#SPJ1
What number should be placed in front of HCl to
balance this chemical equation?
Zn + __HCI - ZnCl2 + H2
Answers
Answer:
it should be 2
Explanation:
Which of the following is a homogenous mixture? a. water b. chicken c. raisin bread d. salad
Answers
B and C are ur answers
Calculate the pH when 90.0 mL of 0.200 M HBr is mixed with 30.0 mL of 0.400 M CH₃NH₂ (Kb = 4.4 × 10⁻⁴).
Answers
The pH of the solution is 10.82.
To solve this problem, we need to determine the concentration of the hydronium ion (\(H_3O^+\)) in the solution. This can be done using the following steps:
Write the balanced chemical equation for the reaction between HBr and CH₃NH₂.
HBr + CH₃NH₂ → CH₃NH₃⁺ + Br⁻
Write the expression for the base dissociation constant (Kb) for CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
Calculate the concentration of hydroxide ions (OH⁻) in the solution using the Kb value and the concentration of CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
4.4 × 10⁻⁴ = x² / (0.400 M)
x = 6.63 × 10⁻³ M
[OH⁻] = 6.63 × 10⁻³ M
Calculate the concentration of \(H_3O^+\) using the equilibrium constant for the reaction between HBr and \(H_2O\).
\(HBr + H_2O = H_3O^+ + Br^-\)
\(Kw = [H_3O^+][OH^-] = 1.0 * 10^{-14}\\[H_3O^+] = Kw/[OH-] = 1.51 * 10^{-11}\)
Calculate the pH using the concentration of \(H_3O^+\).
\(pH = -log[H_3O^+]\\pH = -log(1.51 * 10^{-11})\)
pH = 10.82
For more question on pH click on
https://brainly.com/question/172153
#SPJ11
4. Long answer type questions: a. b. C. d. e. f. g. h. j. i. What are the constituent gases of air? Why is the surrounding air not seen with the eyes? How do you prove that air supports burning? How do you show that air occupies space? How do you prove that air has weight? How is air useful to us? Mention any three points. Write any three properties of air. How can you say that air exerts force? Write any four effects of air pollution. Write any three causes of air pollution and any two control measures of it.
Answers
1. The constituent gases of air are:
Nitrogen Oxygen Argon Carbon Dioxide2. The surrounding air is not seen with the eyes because it is transparent. Air molecules are not visible to the na-ked eye, and they do not scatter or absorb visible light significantly. Therefore, air appears colorless and transparent.
What is air?3. To prove that air supports burning, you can perform an experiment with a burning candle. Place a glass jar or bell jar over a lit candle, ensuring that the jar is airtight. As the candle burns, it consumes oxygen from the air inside the jar. Eventually, the candle flame will go out due to the lack of oxygen, proving that air (specifically oxygen) is necessary for burning.
4. To show that air occupies space, you can perform a simple experiment using a plastic bottle or syringe. Fill the bottle or syringe with water, ensuring there are no air bubbles. Then, cover the opening tightly and try to compress the air inside. You will find that it is not possible to compress the air significantly, indicating that air occupies space.
5. To prove that air has weight, you can use a sensitive balance or scale. Weigh an airtight container or balloon, and then fill it with air. The weight of the container or balloon with the added air will be greater than its initial weight, demonstrating that air has weight.
6. Air is useful to us in various ways. Three points highlighting the importance of air are:
Breathing and RespirationCombustion and Energy ProductionClimate Regulation7. Three properties of air include:
Air is Compressible: Air can be compressed or expanded under different conditions, allowing it to fill various spaces and containers.Air has Mass: Air molecules have mass, which means air itself has weight. It exerts pressure on objects and surfaces.Air Exerts Pressure: Due to the collisions of air molecules with surfaces, air exerts pressure in all directions. This pressure is known as atmospheric pressure.Air exerts force in various ways. For example, air pressure allows objects like airplanes to fly by providing lift. Air resistance or drag opposes the motion of objects moving through the air, creating a force that can affect their speed and trajectory.
8. Four effects of air pollution include:
Respiratory ProblemsEnvironmental Damage:Climate ChangeHuman Health Impacts9. Causes of pollution:
Industrial EmissionsVehicle EmissionsResidential and Agricultural Activities10. Two control measures for air pollution include:
Emission ReductionAir Quality RegulationsLearn more about air on https://brainly.com/question/15215203
#SPJ1
X is a hydrocarbon compound with molecular formula C6H12. X exhibits functional
n
group isomerism with an alkene.
CnH
11
(a) Draw all possible structures of X.
(b) Based on your answer in (2)(a),
(i) identify a pair of chain isomers.
(ii) choose the structures that exist as geometrical isomers and explain your
answer.
(iii) Based on your answer in 3b(ii) draw a pair of geometrical isomer.
[11 Marks]
Answers
Alkene and cycloalkane are the two potential functional group isomers of C6H12. There are six alternative places for the double bond in the alkene, resulting in six different structures.
What are the isomers of its geometry?Three and four hexenes are geometric isomers. While the trans isomer of 3-hexene has the methyl groups on opposing sides of the double bond, the cis isomer of the compound has both methyl groups on the same side.
What is a double bond in carbon?The carbon-carbon double bonds in the structures 1-hexene, 2-hexene, 3-hexene, and 4-hexene prevent rotation around the bonds, allowing them to exist as geometric isomers.
To know more about cycloalkane visit:-
brainly.com/question/30737838
#SPJ1
13 C4- has _______ electrons.
Answers
Answer:
10 electrons
Explanation:
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
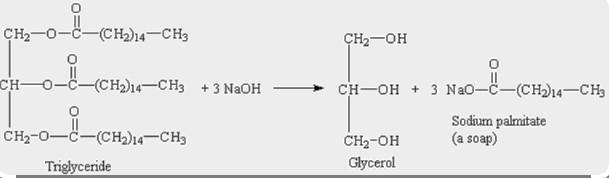
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
How many electrons are in the highest occupied energy level, n, of an element in Group 15?
Answers
Answer:
5 Electrons.
Explanation:
Hello.
In this case, an element in group 15 such as nitrogen has 5 electrons on the outer shell because of its electron configuration. For N, whose atomic number is 7, the electron configuration is:
\(N^7: 1s^2,2s^2,2p^3\)
Whereas energy level 2 has 5 electrons (2 from 2s and 3 from 2p). Next element is phosphorous and its electrons configuration is:
\(P^{15}:1s^2,2s^2,2p^6,3s^2,3p^3\)
It also has 5 electron on the energy level 3 (2 from 3d and 3 from 3p).
Best regards!
Determine the number of moles of phosphorus contained in 1.86 × 10^24 atoms of phosphorus.
___?___ moles P
Answers
1.86e24 atoms / 6.02e23 = 3.09 mol
Which process creates two new cells from one existing cell in order to maintain homeostasis? (4 points) a Cell division b Cellular respiration c Photosynthesis d Reparation
Answers
Answer:
Cell division.
Explanation:
1 points
A bottle contains a mixture of two gases: Oxygen and Hellum. The partial pressure of O2 is 1.0 atm and the partial pressure of He is 100.0 mmHg. What is the total pressure in the tank? (Volume and temperature are
constant)
101 alm
011 atm
O 101 mmHg
O 1.1 mmHg
Answers
101 mmHg is the total pressure in the tank.
Thus, Dalton's Law of Partial Pressures states that while the volume and temperature of a gaseous mixture are held constant, the total pressure of the mixture is equal to the sum of the partial pressures of its gaseous components.
Nitrogen, oxygen, argon, carbon dioxide, water vapor, and a trace amount of other gases are all present in atmospheric air and pressure.
The increased oxygen content in the chamber can displace the CO bound to hemoglobin faster than air oxygen, hence the hyperbaric chambers are also used to treat carbon monoxide (CO) poisoning. The treatment of scuba divers with the bends is another application for hyperbaric chambers.
Thus, 101 mmHg is the total pressure in the tank.
Learn more about Pressure, refer to the link:
https://brainly.com/question/30673967
#SPJ1
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be
true for heat to transfer from sample A to sample B?
O The average kinetic energy of A is greater than that of B.
O The average kinetic energy of B is greater than that of A.
O The average kinetic energy of both samples is equal.
O The average kinetic energy does not determine the direction of heat transfer.
Answers
The direction of heat transfer between two samples of carbon depends on their temperature difference, and not solely on their average kinetic energy. While the average kinetic energy of a substance is related to its temperature, it is not the determining factor for the direction of heat transfer.
When two samples of carbon come into contact, a heat transfer will occur between sample A and sample B. The direction of heat transfer is dependent on the temperature difference between the samples. Heat transfer always flows from a hotter object to a cooler object, so if sample A is hotter than sample B, heat will flow from A to B. If sample B is hotter than sample A, heat will flow from B to A.
The average kinetic energy of the molecules in a substance is related to its temperature. The higher the average kinetic energy, the higher the temperature of the substance. However, the average kinetic energy does not determine the direction of heat transfer.
It is possible for a substance with a lower average kinetic energy (and therefore a lower temperature) to transfer heat to a substance with a higher average kinetic energy (and therefore a higher temperature). This can occur if the substance with the lower temperature has a greater heat capacity, which means it can absorb more heat without a significant increase in temperature.
for more questions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
Which of the following statements is true?
Question 10 options:
a chromosome is larger than a cell
a DNA strand is made of many chromosomes
a gene contains may chromosomes
a chromosome contains many genes
Answers
Answer:
a chromosome contains many genes
Explanation:
How many moles in 3.30g of iron
Answers
The answer below is correct but to give you the process, here it is:
Molar mass of iron, Fe = 55.85 g/mol
3.30g/(55.85 g/mol) = 0.0591 mol
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
Which contains more molecules? 10.0 g of CO2 or 5.60L of oxygen gas at STP
Answers
Answer:
Oxygen.
Explanation:
Hello!
In this case, since the ideal gas equation allows us to compute the moles of oxygen in 5.60 L at STP (1 atm and 273.15 K) as shown below:
\(PV=nRT\\\\n=\frac{PV}{RT}=\frac{1atm*5.60L}{0.08206\frac{atm*L}{mol*K}*273.15K}\\\\n_{O_2}= 0.25mol\)
Next, given the molar mass of carbon dioxide (44.01 g/mol) we compute the moles in 10.0g of this gas via:
\(n_{CO_2}=10.0gCO_2*\frac{1molCO_2}{44.01gCO_2} =0.23molCO_2\)
Thus, since oxygen has the greatest number of moles, we immediately infer it also has the greatest number of molecules based on the Avogadro's number.
Best regards!
When heated, calcium carbonate, CaCO3(s) , decomposes to calcium oxide, CaO(s) , and carbon dioxide, CO2 . Using relevant data from your book's appendices, calculate the heat evolved or consumed when 15.0 g of calcium carbonate are decomposed. answer: kJ
Answers
As per the standard data, the heat evolved during one mole of calcium carbonate decomposes is 177.8 KJ. Thus 15 g or 0.15 moles of calcium carbonate when decomposed will produce 26.67 KJ of heat.
What is reaction enthalpy?Reaction enthalpy of a substance is the heat evolved or absorbed during a reaction. Reaction enthalpy is negative for an exothermic reaction and positive for an endothermic reaction.
Molar mass of calcium carbonate = 100 g.
no.of moles in 15 g = 15 /100 = 0.15 moles.
One mole or 100 g of calcium carbonate decompose to evolve 177.8 KJ according to the scientific record.
Thus, heat evolved by the decomposition of 0.15 moles is 0.15 × 177.8 KJ = 26.67 KJ.
Hence, the heat evolved during the decomposition of 15 g of calcium carbonate is 26.67 KJ.
To find more on reaction enthalpy, refer here:
https://brainly.com/question/1657608
#SPJ1
class material don't interact please
Answers
A 0.00143 M concentration of MnO4^- is not a reasonable solution .
Number of moles of carbonateThe ions left in solution are Na^+ and NO3^-
Number of moles of calcium nitrate = 100/1000 L × 1 = 0.1 moles
Since;
1 mole of sodium carbonate reacts with 1 mole of calcium nitrate then 0.1 moles of sodium carbonate were used.
Conductivity of filtrateThe claim of the student that the concentration of sodium carbonate is too low is wrong because the value was calculated from concentration and volume of calcium nitrate and not using the precipitate. If the filtrate is tested for conductivity, it will be found to conduct electricity because it contains sodium and NO3 ions.
2) In the reaction as shown, the MnO4^- ion was reduced.
The initial volume is 3.4 mL while the final volume is 29.6 mL.
Number of moles of MnO4^- ion = (29.6 mL - 3.4 mL)/1000 × 0.0235 M = 0.0006157 moles
The calculations are performed as followsIf 2 moles of MnO4^- reacted with 5 moles of acid0.0006157 moles of MnO4^- reacted with 0.0006157 moles × 5 moles/ 2 moles
= 0.0015 moles
In this case, number of moles of acid = 0.139 g/90 g/mol = 0.0015 molesNumber of moles of MnO4^- = 0.00143 M × (29.6 mL - 3.4 mL)/1000
= 0.000037 moles
If 2 moles of MnO4^- reacts with 5 moles of acid0.000037 moles of MnO4^- reacts with 0.000037 moles × 5 moles/ 2 moles
= 0.000093 moles
Hence, this is not a reasonable amount of solution.Learn more about MnO4^- : https://brainly.com/question/10887629
Which part of the brain could be damaged if the patient has difficulty in creating and organizing sentences?
Answers
Damage to frontal lobe neurons or tissue can result in personality changes, difficulty concentrating or planning, and impulsivity. The frontal lobe of the brain could be damaged if the patient has difficulty in creating and organizing sentences.
What is frontal lobe ?The frontal lobes are found directly behind the brow. The frontal lobes are the largest lobes in the human brain and the most commonly injured region in traumatic brain injury.
The frontal lobe is in charge of higher cognitive functions such as memory, emotions, impulse control, problem-solving, social interaction, and motor function as a whole.
The frontal lobe of the brain is in charge of high-level cognitive abilities as well as primary motor functions. It is the hub of our personality and communication skills.
Thus, The frontal lobe of the brain could be damaged if the patient has difficulty in creating and organizing sentences.
To learn more about frontal lobe, follow the link;
https://brainly.com/question/14567052
#SPJ1
6 g of metal M react completely with 23.66 g of chlorine to form 29.66 g of the metallic chloride. Find the empirical formula of the metallic chloride. (M = 27, C1 = 35.5) stop'
Answers
Answer:
Below in bold.
Explanation:
6 / 27 = 0.22222..
29.66 / 35.5 = 0.66648
Ratio of M to Cl is 1:6
Empirical formula = MCl3.
02 Question (6 points) 1st attempt IH See Periodic Table Name and define the four quantum numbers that identify the highest energy electron in a francium; Fr, atomic orbital; Symbol Value Principal quantum number Ancular momentum quantum number Magnetic quantum number Spin magnetic quantum number
Answers
The four quantum numbers that identify the highest energy electron in a francium, fr, atomic orbital are n, ℓ, m, and s.
Which four quantum numbers are there?The energy levels of an atom are connected by a set of quantum numbers. The entire and singular quantum state of a single electron in an atom, also known as its wave function or orbital, is described and explained by the four quantum numbers, n, l, m, and s. Accordingly, the four quantum numbers are n, l, m, and s.
The quantum number is used to define the location of electrons, and an orbital is a place inside the atom where an electron can be found. The quantum number of the shell orbitals of an atom depends on the number of electron shells it contains.
Learn more about quantum here: brainly.com/question/5927165
#SPJ4
A scientist studies the effect of adding different amounts of salt on the boiling point of water. He places his results in the
graph below.
What are the independent and dependent variables in this experiment?
Mass is the independent variable, and boiling point is the dependent variable
Bowling point is the independent variable, and mass is the dependent variable
There are two independent variables and no dependent variables
There are two dependent variables and no independent variables
Answers
Answer:
Mass is the independent variable, and boiling point is the dependent variable.
16) Select the best answer.
Round the answer to the correct number of significant figures.
10.05
2.8899 = 29.043495
29.0435
29.04
29.043
29
Answers
29 is not the best answer depends on the context and the rules for significant figures.
What is best answer?
The best answer depends on the context and the rules for significant figures. If we assume that we need to round to three significant figures:
10.05 has three significant figures, so it is already rounded correctly.2.8899 has four significant figures, so we need to round it to three significant figures. The third significant figure is 9, which is greater than 5, so we round up the second significant figure (which is 8) to 9. Therefore, 2.8899 rounded to three significant figures is 2.89.29.0435 has five significant figures, so we need to round it to three significant figures. The third significant figure is 0, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.0435 rounded to three significant figures is 29.0.29.04 has four significant figures, so it is already rounded correctly.29.043 has four significant figures, so we need to round it to three significant figures. The third significant figure is 3, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.043 rounded to three significant figures is 29.0.29 has one significant figure, so it is not rounded correctly to three significant figures.Therefore, 29 is not the best answer.
To know more about significant figures, visit:
https://brainly.com/question/30465808
#SPJ1