Within the human body the most abundant chemical compound is.
Answers
Answer:
oxygen is the most chemical compounds
Related Questions
find the oxidation number of carbon in MgCO3
Answers
Answer: +2
Explanation: Formula of oxide is MgO, because oxidation state of Mg in MgCO3 is +2.
ANSWER THIS QUICKLY FOR BRAINLIEST!!

Answers
Answer:
do good
Explanation:
do good
The blanks and bottom part please!
Thank you in advance

Answers
The complete sentences are:
When all the intermolecular bonds are broken, the transition between phases is complete.The energy of any substance includes the kinetic energy of its particles and the potential energy of the bonds between its particles.What are the complete sentences on matter?Page 3:
The effect of energy in phase transitions of matter is that it is required to break the intermolecular forces that hold the particles of a substance together. When energy is added to a substance, the particles move faster and the intermolecular forces are broken. This can cause the substance to change phase.
The interactive demonstration on the sample of water shows that energy is required to melt ice and boil water. When the ice is heated, the particles start to move faster and the ice melts. The temperature of the water stays constant at 0°C until all of the ice has melted. This is because the energy is being used to break the intermolecular forces in the ice. Once all of the ice has melted, the temperature of the water starts to rise again. When the water is boiled, the particles move so fast that they escape from the liquid state and become a gas. The temperature of the water stays constant at 100°C until all of the water has boiled. This is because the energy is being used to break the intermolecular forces in the water. Once all of the water has boiled, the temperature of the steam starts to rise again.
The complete sentences:
Water stays in a liquid state as the temperature and kinetic energy of the molecules increase from 0°C to 100°C. This consistency indicates that a larger amount of energy is necessary to break the intermolecular forces and change the state of matter. At the melting and boiling points, the temperature does not change because all of the energy is being used to break the intermolecular forces.The energy needed to overcome all the intermolecular forces between molecules must be greater than the potential energy of the bonds between molecules.The transition between phases is a physical change, not a chemical change.Page 4:
Heating curves show the temperature of a substance as it is heated. The curve has a horizontal line at the melting and boiling points, which indicates that the temperature does not change during these phase changes.
Cooling curves show the temperature of a substance as it is cooled. The curve has a horizontal line at the melting and boiling points, which indicates that the temperature does not change during these phase changes.
Both curves show that the temperature of a substance increases as it is heated and decreases as it is cooled.
A heating curve is more choppy than a cooling curve because there are more phase changes during heating than during cooling.
Find out more on matter here: https://brainly.com/question/3998772
#SPJ1
How does inclusions represent the principles
Will give 15 points
Answers
PLZ HELP ME WITH MY WORK

Answers
Answer:
It should be the answer D. A type of atom
Explanation:
A model is a "synthetic coordination entity that closely approaches the properties of a metal ion in a protein"
An ion is a charged atom.
Select all the correct answers.
Which observation shows that a change in state of matter has occurred?
A) Wood burns, producing gas and an ashy residue.
B) Overnight, dew forms on the grass.
C) An alkali indicator changes color when added to a cleaner.
PLEASE BE ACCURATE!! THANK YOU!!:)
Answers
The observation that shows a change in state of matter has occurred is option A) Wood burns, producing gas and an ashy residue.
A change in state of matter refers to the transformation of a substance from one physical state to another, such as from solid to liquid, liquid to gas, or vice versa. In option A, when wood burns, it undergoes a chemical reaction known as combustion.
During combustion, the wood reacts with oxygen in the air and undergoes a transformation from a solid state to a gaseous state, producing gas (such as carbon dioxide and water vapor) as well as an ashy residue (solid carbon and other impurities remaining after the combustion process). This change in state is accompanied by the release of heat and light.
Option B, where dew forms on the grass overnight, does not indicate a change in state of matter. Dew formation is a physical process known as condensation, where water vapor in the air cools down and changes into liquid water droplets upon contact with a colder surface (such as grass). In this case, the state of matter remains the same (from a gaseous state to a liquid state), and no new substances are formed.
Option C, where an alkali indicator changes color when added to a cleaner, also does not represent a change in state of matter. It suggests a chemical reaction between the alkali indicator and the cleaner, resulting in a color change. However, the states of matter involved (liquid or solid) do not change during this process.
In summary, option A is the observation that indicates a change in state of matter, as it involves the transformation of wood from a solid state to a gaseous state during combustion.
For more such question on matter visit:
https://brainly.com/question/1172629
#SPJ8
find the volume. mass is 9.75 g and the density is 15.6 g/cm3
Answers
Answer:
1.6cm3
Explanation:
density = mass/volume
15.6g/cm3= 9.75 g/volume
15.6/9.75 = volume
1.6cm3 = volume
what are the properties of acids
Answers
Explanation:
Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current.
Acids have a sour taste.
Acids change the color of certain acid-base indicates.
Acids react with active metals to yield hydrogen gas.
Acids react with bases to produce a salt compound and water.
what is diffrence bettwen thermal energy and heat
Answers
Answer:
Thermal, is mainly the heat trapped or in an object, such as a boiling water over a stove, its trapped and countinues to get hotter
Heat is basically the releasing of the energy that is contained in an object.
Thermal is the objects temperature, while heat is the Thermal energy that is released.
Explanation:
. The model shows Earth in several positions relative to the Sun. In which position is it winter in North America?
Answers
what is the trend in dielectric constants for the following solvents? group of answer choices ccl4 > acetone > dmso h2o < acetone < hexane h2o > acetone > hexane dmso > acetone > ccl4
Answers
The answer is DMSO > Acetone > CCl₄ .
An indicator of a substance or material's capacity to store electrical energy is its dielectric constant. It is a measurement of how much an object can hold or concentrate an electric flux.
Dielectric constant is defined mathematically as the ratio of a material's permittivity to the permittivity of free space. It also goes by the name relative permittivity for this reason. It is relative magnetic permeability's electrical equivalent.
here, the dielectric constant of Acetone is 20.7 , CCl₄ ( Carbon tetrachloride) is 2.23 and DMSO ( Dimethyle Sodium Oxide ) is 46.68.
So, the trend in dielectric constant for Acetone, CCl₄ and DMSO is,
DMSO ( 46.68 ) > Acetone ( 20.7 ) > CCl₄ ( 2.23 ) .
To learn more about Solvents :
https://brainly.com/question/21314456?referrer=searchResults
#SPJ4
50 mL of 0.60 M sodium hydroxide neutralized 20 mL of sulfuric acid. Determine the concentration of the acid.
Answers
2NaOH + H2SO4 → Na2SO4 + 2H2O
From the balanced equation, we can see that 2 moles of NaOH react with 1 mole of H2SO4.
The number of moles of NaOH used is:
0.050 L x 0.60 mol/L = 0.030 mol
Since 2 moles of NaOH react with 1 mole of H2SO4, the number of moles of H2SO4 in 20 mL of solution is:
0.030 mol NaOH x (1 mol H2SO4 / 2 mol NaOH) = 0.015 mol H2SO4
The concentration of the sulfuric acid is:
0.015 mol / 0.020 L = 0.75 M
A chemistry student needs to standardize a fresh solution of sodium hydroxide. She carefully weighs out 197. mg of oxalic acid (H, C04), a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250. mL of distilled water. The student then titrates the oxalic acid solution with her sodium hydroxide solution. When the titration reaches the equivalence point, the student finds she has used 45.3 mL of sodium hydroxide solution.
Calculate the molarity of the student's sodium hydroxide solution.
Answers
The molarity of the student's sodium hydroxide solution is 0.0689 M.
To determine the molarity of the sodium hydroxide solution, we can use the stoichiometry of the balanced equation between sodium hydroxide (NaOH) and oxalic acid (H2C2O4).
The balanced equation for the reaction between NaOH and H2C2O4 is:
2NaOH + H2C2O4 → Na2C2O4 + 2H2O
From the balanced equation, we can see that the ratio of NaOH to H2C2O4 is 2:1. This means that for every 2 moles of NaOH, 1 mole of H2C2O4 is consumed.
Given that the student used 45.3 mL of NaOH solution, we need to convert this volume to moles of NaOH. To do this, we need to know the molarity of the oxalic acid solution.
Using the given mass of oxalic acid (197 mg), we can calculate the number of moles of H2C2O4:
moles of H2C2O4 = mass of H2C2O4 / molar mass of H2C2O4
The molar mass of H2C2O4 is 126.07 g/mol.
moles of H2C2O4 = 0.197 g / 126.07 g/mol = 0.001561 mol
Since the stoichiometry of the reaction is 2:1, the number of moles of NaOH used is twice the number of moles of H2C2O4:
moles of NaOH = 2 * moles of H2C2O4 = 2 * 0.001561 mol = 0.003122 mol
Now we can calculate the molarity of the NaOH solution:
Molarity of NaOH = moles of NaOH / volume of NaOH solution in liters
Volume of NaOH solution = 45.3 mL = 45.3/1000 L = 0.0453 L
Molarity of NaOH = 0.003122 mol / 0.0453 L = 0.0689 M.
For more such questions on molarity visit:
https://brainly.com/question/30404105
#SPJ8
Both artists and scientists contribute to our understanding of the world around us. Identify the ways that art and science are similar. Check all that apply.
Both artists and scientists use their imagination to create things.
Both artists and scientists rely on others in their field for inspiration.
Both artists and scientists can modify their work whenever necessary.
Both artists and scientists use technological innovations to explore new possibilities.
Answers
Arts helps us to understand the world with the help of textures and deepness of the art. Science is the only way with the help of which we are able to understand the beauty of the nature.
How artists and scientists help us to understand the world around us?
Arts:
Arts gives us meaning and help us to understand the world around us. Even the Scientific studies have proven that art appreciates improves our life quality of life and makes us feel good. When we create the art then we define our mood, the situations around us, the environment exists around us and open our minds with new ideas.
Science:
First of all the science helps our understanding of the world around us. Every thing we know about the universe, we know how the trees grew up, we know how the habitat exists on the earth, we know that how the weather and climate changes. This is all due to the science. Human progress throughout history has largely rested on advances in science.
So we can conclude that: Arts helps us to understand the world with the help of textures and deepness of the art. Science is the only way with the help of which we are able to understand the beauty of the nature.
Learn more about Art here: https://brainly.com/question/5085700
#SPJ1
Answer:
A: Both artists and scientists use their imagination to create things.
B: Both artists and scientists rely on others in their field for inspiration.
D: Both artists and scientists use technological innovations to explore new possibilities.
Explanation:

You have prepared a 1.0 M solution of CaCl2 in the laboratory.
What is the concentration of chloride ions in the solution?
a. 0.50 M
b. 1.0 M
c. 1.5 M
d. 2.0 M
Answers
You have prepared a 1.0 M solution of \(CaCl2\) in the laboratory the concentration of chloride ions in the solution is 1.5 M.
What is solution?Solution is defined as huge amount of solute dissolved in solvent. The proportion of solute is limited and after reaching at the point of saturation no more solute will be mixed in the solvent. It is impossible to separate solvent and solute from eye.
Pressure plays a important role in rate of reaction to increase the pressure it increases the rate of reaction by increasing the collision.
Pressure increase the concentration of gases it means that it increases the number of molecules per unit volume due to this collision of gases increases this will increase the temperature. This increase of temperature will increase the rate of reaction.
Therefore, you have prepared a 1.0 M solution of CaCl2 in the laboratory the concentration of chloride ions in the solution is 1.5 M.
Learn more about solution here:
https://brainly.com/question/7932885
#SPJ2
In a new compound, it is found that the central carbon atom is sp2 hybridized. This implies that.
Answers
If the central metal ion is sp² hybridized in the new atom, it means that bond of carbon are formed by hybridization of 2 s orbitals and 1 p orbital.
Hybridization means that there is not a singular orbital, there is a new orbital which is forced by the hybridization of the old orbital.
It also means that the central carbon atom is an alkene. it will have a double bond.
It will have two sigma bonds and one pi bond. The bonding in the sp² orbital will have the electron in the s orbital and the p orbital while the carbon atom is in the excited state. Here, s orbitals will be forming two sigma bond and p orbital will be forming the pi bond.
To know more about hybridization, visit,
https://brainly.com/question/22765530
#SPJ4
Chemistry problem, I’m conflicted between A or D

Answers
If the balloons are placed in a warmer room, all of the balloons will increase in volume equally because they have equal numbers of molecules.
The correct answer is D.
What happens to the volume of gases when they are heated?According to the ideal gas law, the volume of a gas is directly proportional to its temperature when the pressure and number of moles are held constant.
When the balloons are placed in a warmer room, the temperature increases resulting in an increase in volume. Since all three balloons have the same number of molecules and experience the same increase in temperature, they will all increase in volume equally.
Learn more about the volume of gases at: https://brainly.com/question/25736513
#SPJ1
When you add water to a strong acid what happens to the pH of the solution
Answers
Hope this helpss and good luck on y’alls examssss :)))
NO ONE IS ANSWERING PLS HELP!
Which product in the reaction forms a precipitate?
AgCl
AgK
No precipitate formed

Answers
Answer:
AgCl
Explanation:
Got correct on E2020
Answer:
B: AgCl
Source: trust me bro
Which structure below represents the enantiomers of the following compound on the picture there can be multiple options
Answers
Answer:
To get enatiomer of the given compound the R-S COMBINATION WILL BE. RRS OF THE BELOW COMPOUND.NOW, FOR A, IT IS SRS SO IT IS DIESTEREOMER.FOR B,D,F IT WILL BE SSR SO, B,D,F WILL BE ENANTIOMERS.FOR E,C IT WILL BE SSR .SO, THEY ARE IDENDICAL.SO, B+D+F WILL BE ANS.
Explanation:
For the reaction Cl2O + 3/2 O2 (g) <--> 2 ClO2 .... Delta H = 126 kJ/mol. Delta S = -74.9 J/Mol K at 298 K. What is the Keq? Answer: 1.0 x 10-26.
2. O3 (g) + 2NO2 (g) --> O2 (g) + N2O5 (g)
(1) O3 + NO2 <--> O2 +NO3 (fast equil.)
(2) NO3 + NO2 --> N2O5 (slow)
What is the rate law of the above reaction?
Answers
The equilibrium constant (Keq) for the reaction Cl₂O + 3/2 O₂ (g) ⇌ 2 ClO₂ at 298 K is 1.0 x 10⁻²⁶.
Determine the equilibrium constant?The equilibrium constant (Keq) is determined using the equation Keq = \(\rm e^{(-\Delta G/RT)\), where ΔG is the standard free energy change, R is the gas constant, and T is the temperature in Kelvin. However, in this case, we are given ΔH (enthalpy change) and ΔS (entropy change), so we need to use the equation ΔG = ΔH - TΔS.
Plugging in the given values:
ΔH = 126 kJ/mol = 126,000 J/mol
ΔS = -74.9 J/mol K
T = 298 K
ΔG = ΔH - TΔS
ΔG = 126,000 J/mol - 298 K * (-74.9 J/mol K)
ΔG = 126,000 J/mol + 22,320 J/mol
ΔG = 148,320 J/mol
Now, we can calculate Keq:
Keq = \(\rm e^{(-\Delta G/RT)\)
Keq = \(\rm e^{(-148,320\ J/mol) / (8.314\ J/mol K \times 298\ K))}\)
Keq ≈ 1.0 x 10⁻²⁶
Therefore, At a temperature of 298 K, the equilibrium constant (Keq) for the reaction between Cl₂O and 3/2 O₂ to form 2 ClO₂ is approximately 1.0 x 10⁻²⁶.
To know more about equilibrium constant, refer here:
https://brainly.com/question/29809185#
#SPJ4
Why must the cyclopentadiene be freshly distilled and kept cold immediately prior to the Diels-Alder rea Select all that apply.
All Diels-Alder reactions must be performed hot.
Cyclopentadiene dimerizes at room temperature.
Heating reverses the Diels-Alder reaction of cyclopentadiene with itself.
All Diels-Alder reactions must be performed cold.
Answers
Answer:
- Cyclopentadiene dimerizes at room temperature.
- Heating reverses the Diels-Alder reaction of cyclopentadiene with itself.
Explanation:
Cyclopentadiene dimerizes at room temperature, and heating reverses the Diels-Alder reaction of cyclopentadiene with itself. Therefore, cyclopentadiene must be freshly distilled and kept cold immediately prior to the Diels-Alder reaction.
So the correct options are:
Cyclopentadiene dimerizes at room temperature.
Heating reverses the Diels-Alder reaction of cyclopentadiene with itself.
please mark brainliest thanks have a great day :)
The options which apply are Cyclopentadiene dimerizes at room temperature. Heating reverses the Diels-Alder reaction of cyclopentadiene with itself.
In the context of the Diels-Alder reaction, there are a few key reasons why fresh and cold cyclopentadiene is preferred:
Cyclopentadiene dimerizes at room temperature:
Cyclopentadiene is a very reactive molecule and can easily undergo dimerization to form dicyclopentadiene, especially at room temperature or higher. Dicyclopentadiene is an unreactive solid that can hinder the Diels-Alder reaction or lead to side reactions. Freshly distilled cyclopentadiene is less likely to contain dimerization products and therefore more reactive.
The Diels-Alder reaction is exothermic:
The Diels-Alder reaction between cyclopentadiene and dienophiles is exothermic, meaning it releases heat. If the reaction is performed at high temperatures, the reaction can become too vigorous, leading to unwanted side reactions or decomposition of the reactants. Keeping the reactants cold can help control the reaction and prevent runaway heating.
Heating reverses the Diels-Alder reaction of cyclopentadiene with itself:
Cyclopentadiene can undergo a Diels-Alder reaction with itself to form dicyclopentadiene, but this reaction is reversible. Heating can cause the dicyclopentadiene to break down back into cyclopentadiene, leading to a decrease in the yield of the desired Diels-Alder product. Keeping the cyclopentadiene cold can help prevent this reverse reaction.
Click the below link, to learn more about Cyclopentadiene:
https://brainly.com/question/12995682
#SPJ11
what is the molarity of kmno4 in a solution of 0.0897 g of kmno4 in 0.450 l of solution?
Answers
The molarity of KMnO4 in the given solution is approximately 0.00126 M. Molarity is a measure of the concentration of a solute in a solution. It represents the number of moles of the solute present per liter of the solution.
To determine the molarity of KMnO4 in the given solution, we need to first calculate the number of moles of KMnO4 using its mass and molar mass, and then divide it by the volume of the solution.
The molar mass of KMnO4 can be calculated as follows:
(1 × atomic mass of potassium) + (1 × atomic mass of manganese) + (4 × atomic mass of oxygen)
= (1 × 39.10 g/mol) + (1 × 54.94 g/mol) + (4 × 16.00 g/mol)
= 39.10 g/mol + 54.94 g/mol + 64.00 g/mol
= 158.04 g/mol
Now, let's calculate the number of moles of KMnO4:
Number of moles = Mass / Molar mass
Number of moles = 0.0897 g / 158.04 g/mol
Number of moles ≈ 0.000567 mol
Next, we need to calculate the molarity using the number of moles and the volume of the solution:
Molarity (M) = Number of moles / Volume (in liters)
Molarity = 0.000567 mol / 0.450 L
Molarity ≈ 0.00126 M
In this case, the molarity tells us the concentration of KMnO4 in moles per liter.
Learn more about potassium at: brainly.com/question/13321031
#SPJ11
matter graphic organizer
Answers
Please answer question #4
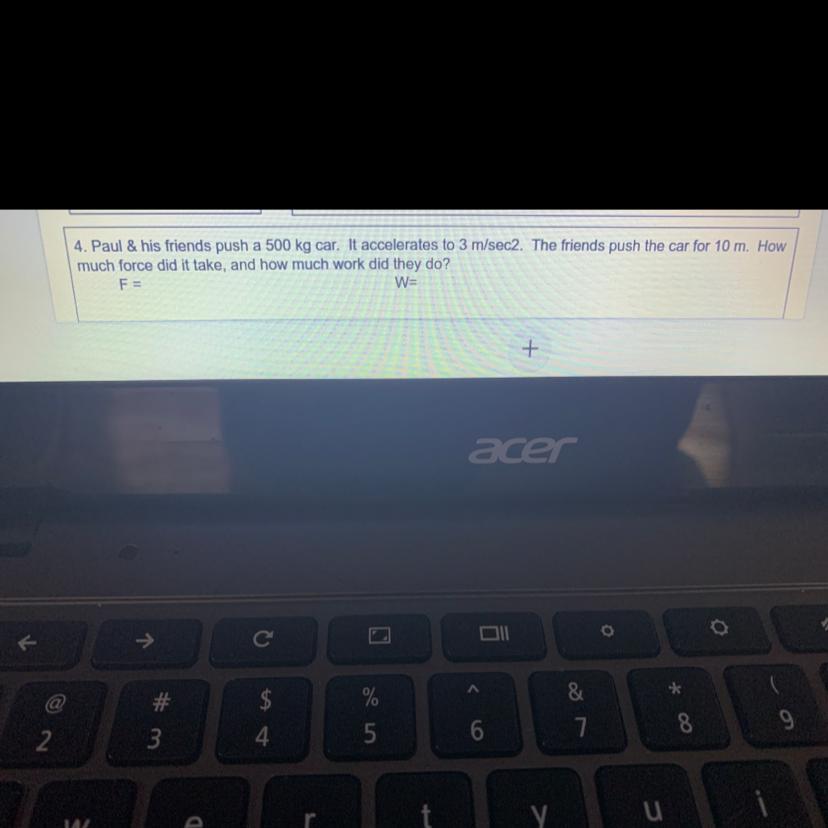
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
How is a chemical equation different from a chemical formula?
Answers
Answer:
Explanation:
A chemical formula is the combination of atomic symbols that designate a particular chemical compound, or a substance with two or more different elements. The formula shows which type and how many of each element makes up that specific compound
Which of these equations for the
formation of an oxide ion is correct?
0 + 2e" --> 02-
0 --> 02- + 2e
Answers
Answer:
A
Explanation:
Because of the cathode
If 48.42 g of copper (II) chloride (CuCl2) reacts with 20.40 g of aluminum metal (Al) to produce 21.00 g of copper metal (Cu). How many moles of which excess reactant remains at the end of the reaction
Answers
At the end of the reaction, 0.5161 mol of aluminum remains in excess.
To determine the excess reactant and the amount remaining at the end of the reaction, we need to calculate the theoretical amount of each reactant required to completely react and compare it to the given amounts.
First, let's calculate the molar mass of each compound:
- Copper (II) chloride (CuCl₂):
- Copper (Cu) has a molar mass of 63.55 g/mol.
- Chlorine (Cl) has a molar mass of 35.45 g/mol.
- Multiply the molar mass of chlorine by 2 since there are two chlorine atoms in the compound:
- 35.45 g/mol × 2 = 70.90 g/mol.
- The molar mass of CuCl₂ is:
- 63.55 g/mol + 70.90 g/mol = 134.45 g/mol.
- Aluminum (Al):
- Aluminum has a molar mass of 26.98 g/mol.
Next, let's calculate the number of moles for each reactant using the given masses:
- Moles of CuCl₂:
- Moles = mass / molar mass
- Moles = 48.42 g / 134.45 g/mol = 0.3600 mol (rounded to four decimal places).
- Moles of Al:
- Moles = mass / molar mass
- Moles = 20.40 g / 26.98 g/mol = 0.7561 mol (rounded to four decimal places).
The balanced chemical equation for the reaction is:
2 Al + 3 CuCl₂ → 3 Cu + 2 AlCl₃
According to the stoichiometry, 2 moles of Al react with 3 moles of CuCl₂ to produce 3 moles of Cu. Therefore, the theoretical amount of CuCl₂ required to react with 0.7561 moles of Al is:
0.7561 mol Al × (3 mol CuCl₂ / 2 mol Al) = 1.134 mol CuCl₂
Similarly, the theoretical amount of Al required to react with 0.3600 moles of CuCl₂ is:
0.3600 mol CuCl₂ × (2 mol Al / 3 mol CuCl₂) = 0.2400 mol Al
To find the excess reactant, we compare the actual amounts given with the theoretical amounts calculated above.
Excess reactant:
- CuCl₂:
- The actual amount of CuCl₂ given is 0.3600 mol, which is equal to the theoretical amount required. Therefore, there is no excess CuCl₂.
- Al:
- The actual amount of Al given is 0.7561 mol, and the theoretical amount required is 0.2400 mol.
- Excess moles of Al = actual moles - theoretical moles
- Excess moles of Al = 0.7561 mol - 0.2400 mol = 0.5161 mol
Therefore, at the end of the reaction, 0.5161 mol of aluminum remains in excess.
Learn more about molar mass, here:
https://brainly.com/question/31545539
#SPJ4
What happens to a reaction as the reactants are used up?A. A different path is taken by the reaction.B. The enthalpy of the reaction changes.C. The equilibrium position changes.D. The rate of the reaction slows down.
Answers
When we have a chemical reaction, the speed of the reaction will depend on different factors such as concentration, temperature, or pressure.
If we assume that the temperature and pressure remain constant, it will be the concentration that will determine the rate of reaction for a non-zero order reaction.
If the concentration of the reactants decreases, the reaction rate also decreases, therefore, if the reactants are depleted, the reaction rate decreases.
Answer: D. The rate of the reaction slows down.
How would you compare dissolving and dispersing waste materials?Dissolving them means flushing them with water; dispersing them means breaking them downDissolving them means breaking them down; dispersing them means recycling themDissolving them means breaking them down; dispersing them means spreading them outDispersing them means cleansing them; dissolving them means flushing them with water
Answers
Dissolving and dispersing are two different approaches to waste disposal. Dissolving is the method of separating and breaking down solid waste materials into smaller particles by using a solvent such as water.
Dispersing involves distributing and breaking up the waste into smaller pieces in order to make it more manageable to dispose of.
Dissolving means breaking them down; dispersing means spreading them out is the right choice.In conclusion, dissolving and dispersing waste materials have different methods. Dissolving refers to the separation and breaking down of solid waste materials into smaller particles by utilizing a solvent such as water.
Dispersing, on the other hand, involves distributing and breaking up waste into smaller pieces to make it more manageable to dispose of.
To know more about waste disposal visit:
https://brainly.com/question/30944259
#SPJ11