Write a balanced equation for the overall reaction (including cyt c oxidation and ATP synthesis).
a. Cytcox+12O2+ATP+2H+⟶Cytcred+ADP+Pi+2H2O
b. 2Cytcred+12O2+2H+⟶2Cytcox+H2O
c. 2Cytcred+12O2+ADP+Pi+2H+⟶2Cytcox+ATP+2H2O
d. Cytcox+O2+4H+⟶Cytcred+2H2O
Answers
The balanced equation for the overall reaction (including cyt c oxidation and ATP synthesis) is:
2Cytcred + 12O2 + 2ADP + 2Pi + 4H+ ⟶ 2Cytcox + 2ATP + 8H2O
This equation shows the oxidation of two molecules of cyt c (Cytcred) and the reduction of twelve molecules of oxygen (O2) to form two molecules of oxidized cyt c (Cytcox), two molecules of adenosine triphosphate (ATP), and eight molecules of water (H2O). The ATP is formed through the process of oxidative phosphorylation, which occurs in the electron transport chain of cellular respiration.
The protons (H+) involved in the reaction are pumped across the inner mitochondrial membrane, creating a gradient that is used to power the synthesis of ATP. The equation is balanced in terms of both atoms and charges, with two electrons being transferred from each cyt c molecule to each oxygen molecule.
To know more about oxidative phosphorylation:
https://brainly.com/question/29104695
#SPJ11
The balanced equation for the overall reaction, which includes the oxidation of cytochrome c (Cyt c) and the synthesis of ATP is c. 2Cytcred+1/2O2+ADP+Pi+2H+⟶2Cytcox+ATP+2H2O
This equation represents the overall process of oxidative phosphorylation, which occurs in the mitochondria of eukaryotic cells. During this process, electrons are transferred from NADH and FADH2 to a series of electron carriers, including cytochrome c, in the electron transport chain. This transfer of electrons creates a proton gradient across the mitochondrial inner membrane, which is then used by ATP synthase to generate ATP from ADP and Pi.
The balanced equation includes the oxidation of two molecules of cytochrome c (2Cytcred) by 12 molecules of oxygen (12O2), as well as the simultaneous synthesis of ATP from ADP and Pi. The equation also includes the consumption of two hydrogen ions (2H+) and the production of two molecules of water (2H2O).
To learn more about the balanced equation, refer:-
https://brainly.com/question/20371395
#SPJ11
Related Questions
PLZ HELP ME Which of the following accurately descnbes the function of the nervous system? O
A To help parts of the body communicate
B. To prevent and fight disease C:
To allow the body to move O
D. To provide support for the body SUNT
Answers
Answer:
A
Explanation:
the nervous system helps parts of the body communicate.through nerves.
I hope this helps
If a substance can be given a chemical formula can be considered to be a(n) __________.
Answers
If a substance can be given a chemical formula can be considered to be a(n) compound.
What is a compound?A pure substance made up of two or more different elements combined chemically in a fixed ratio is called a compound.
Compounds are substances composed of different elements bonded chemically; only chemical reactions break the chemical bonds or create new chemical bonds leading a compound to form other substances.
Hence, if a substance can be given a chemical formula can be considered to be a(n) compound.
Learn more about the compound here:
https://brainly.com/question/13516179
#SPJ1
Which of the following is characteristic of non-metals?
A)
They're always solids at room temperature.
B)
They're strong conductors of electricity.
C)
They tend to be denser than metals.
D)
They tend to gain electrons in chemical reactions.
Answers
Answer:
B
Explanation:
Non metals do not conduct electricity
Answer:
D.
Explanation:
I saw it in the textbook
4. How do chemical processes result in thermal energy being released or absorbed? explain
Answers
Answer:
there's no answer :)
Explanation:
When chemical bonds are formed, heat is released, and when chemical bonds are broken, heat is absorbed
A catalyst that has a different phase than the reactants is a(n) _______. A. exergonic catalyst B. endergonic catalyst C. homogeneous catalyst D. heterogeneous catalyst E. autocatalyst
Answers
Answer: Heterogeneous Catalyst
Explanation: The word heterogeneous means "different phases" or insoluble in science.
10. When carrying out the
experiment with magnesium
and sulphuric acid, why do you
add magnesium until it stops
fizzing?
Answers
Answer:
To make sure all the sulphuric acid has been used up
The boiling point of ethanol is 78. 3 degrees Celsius. The boiling point of
ethanol on the Kelvin scale is approximately. *
Answers
The boiling point of ethanol on the Kelvin scale is (273.15+78.3) = 351.45 approximately.
International consensus establishes the ice point and steam point as the two fixed points that make up the Celsius temperature scale. The steam point is established at 100° Celsius, while the ice point is 0° Celsius.
The proportion of 1/273.15 of the thermodynamic temperature of water's triple point is the definition of a Kelvin. The Kelvin is also known as the thermodynamic temperature scale.
As a unit for a unit, both scales are connected. A change of one unit on the Kelvin scale corresponds to a change of one degree on the Celsius scale. These two scales are identical save from the thermometer zero points.
As a result, the conversion formula is: add 273.15 to convert degrees Celsius to Kelvin, and remove 273.15 to convert Kelvin to degrees Celsius.
Find out more about temperature scale
brainly.com/question/28938811
#SPJ4
What are the ways that energy
conversion can occur?
Answers
Answer:
Energy can exist in many forms within a system and may be converted from one form to another within the constraint of the conservation law. These different forms include gravitational, kinetic, thermal, elastic, electrical, chemical, radiant, nuclear, and mass energy.
Explanation:
help pls:))!!!!!!!!!!

Answers
Answer: C
Explanation:
name the five methods used for the formation of salts??
Answers
Explanation:
In chemistry, a salt is a chemical compound consisting of an ionic assembly of cations and anions.[1] Salts are composed of related numbers of cations (positively charged ions) and anions (negatively charged ions) so that the product is electrically neutral (without a net charge). These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate (CH
3CO−
2); and can be monatomic, such as fluoride (F−) or polyatomic, such as sulfate (SO2−
4).
the solvolysis of t-butyl bromide in methanol yields t-butyl methyl ether in an sn1 reaction (among other products). what is the effect on the rate of reaction of doubling the concentration of t-butyl bromide and quadrupling the concentration of methanol?
Answers
The effect on the rate of reaction for this SN1 process when doubling the concentration of t-butyl bromide is an increase by a factor of 2, while quadrupling the concentration of methanol will have no impact on the rate.
In the solvolysis of t-butyl bromide in methanol, t-butyl methyl ether is produced through an SN1 reaction. When discussing the rate of an SN1 reaction, it's important to note that it is a two-step process involving the formation of a carbocation intermediate. The rate-determining step (RDS) is the first step, which involves the ionization of t-butyl bromide to form a carbocation and a bromide ion.
The rate of an SN1 reaction is directly proportional to the concentration of the substrate (t-butyl bromide) and independent of the concentration of the nucleophile (methanol). Thus, doubling the concentration of t-butyl bromide will double the rate of reaction, as it increases the availability of the substrate for ionization.
On the other hand, quadrupling the concentration of methanol will not affect the rate of the reaction, as it is not involved in the RDS. Methanol reacts with the carbocation in the second step, which is a fast step and does not determine the overall rate.
For more such questions on t-butyl bromide, click on:
https://brainly.com/question/24244711
#SPJ11
A leaking underground storage tank or wastewater pouring into a river from a pipe would be examples of
Answers
Answer:
Water pollution.
Explanation:
This is because water pollution refers to the release or disposal of harmful substances called contaminants which contaminate water bodies which include rivers, oceans, lakes e.t.c.. These harmful substances affect aquatic organisms and make water unfit for good use. This can lead to the death if aquatic lives in the water bodies, release of bad smells and make the water bad . A leaking underground storage tank or wastewater pouring into a river from a pipe can be a source of water pollution.
if 35.22 ml of naoh solution completely neutralizes a solution containing 0.544 g of khp, what is the molarity of the naoh solution?
Answers
The molarity of the NaOH solution is 0.0754 M. Answer: 0.0754 M.
Molarity can be defined as the number of moles of solute present in per liter of solution. To calculate the molarity of the NaOH solution, we need to use the given information. Given that 35.22 mL of NaOH solution completely neutralizes a solution containing 0.544 g of KHP.We can use the formula for molarity:
Molarity = (mass of solute / molar mass of solute) / volume of solution in L
First, we need to calculate the number of moles of KHP.Number of moles of KHP = mass of KHP / molar mass of KHP
Number of moles of KHP = 0.544 / 204.22 = 0.00266 mol
Now, we can use the balanced chemical equation for the reaction between NaOH and KHP:
NaOH + KHC8H4O4 → KNaC8H4O4 + H2O
From the equation, we can see that one mole of NaOH reacts with one mole of KHP. Therefore, the number of moles of NaOH used in the reaction is also 0.00266 mol.Since the volume of the NaOH solution used is 35.22 mL, we need to convert it into liters.Volume of NaOH solution used = 35.22 mL = 0.03522 L
Now we can calculate the molarity of the NaOH solution:
Molarity = number of moles / volume of solution
Molarity = 0.00266 / 0.03522
Molarity = 0.0754 M
Therefore, the molarity of the NaOH solution is 0.0754 M. Answer: 0.0754 M.
To know more about molarity visit:
https://brainly.com/question/31545539
#SPJ11
how many atoms are contained in a 4.65 g sample of the (atomic mass = 4.003 g/mol)?
Answers
Atomic mass of the element = 4.003 g/mol.
The number of atoms in a sample can be calculated using the following formula:
Number of moles = Mass of sample / Molar massAvogadro's number .
Number of atoms = Number of moles × Avogadro's number
Let's solve the problem by substituting the given values in the above formulas:
Given,Mass of the sample = 4.65 g
Atomic mass of the element = 4.003 g/molMolar mass of the element = Atomic mass in g/mol = 4.003 g/molNumber of moles = Mass of sample / Molar mass= 4.65 g / 4.003 g/mol= 1.162 molAvogadro's number = 6.022 × 10²³Number of atoms = Number of moles × Avogadro's number= 1.162 mol × 6.022 × 10²³= 6.99 × 10²³ atoms
Hence, there are 6.99 × 10²³ atoms present in a 4.65 g sample of the element.
Learn more about Atomic mass:
https://brainly.com/question/30390726
#SPJ11
Which of the following most likely happens when thermal energy is removed from a chemical reaction?
Answers
Answer:
fewer collisions occur between particles or lowering the temperature
Explanation:
What does the VSEPR theory describe
Answers
Answer:
The shape of a molecule based on the number of electron pairs on the valence shell of its central atom
Explanation:
The improvement of the Sidgwick-Powell theory came to be known as the Valence Shell Electron Pair Repulsion theory (VSEPR). This theory approaches the determination of molecular shape from the perspective of the number of electron pairs on the valence shell of the central atom in the molecule.
Electron pairs on the valence shells of atoms leads to repulsion. Repulsion between two lone pairs is greater than repulsion between a lone pair and a bond pair which is also greater than repulsion between two bond pairs.
Lone pairs cause more repulsion, hence they distort molecules from the ideal shape predicted based on their electron domain geometry.
Which term is best described as an organic compound containing a carbonyl group bound to two hydrogen atoms or to a hydrogen atom and an alkyl substituent
Answers
A carbonyl group is bound to hydrogen bond or to a hydrogen ion and an alkoxy substituent in an organic compound known as an aldehyde.
What is Aldehyde?Aldehyde is an organic compound containing a functional group with the structure −CHO, in which a carbon atom is double-bonded to an oxygen atom and single-bonded to a hydrogen atom. Aldehydes are the simplest type of carbonyl group and the most common type of organic compound. Aldehydes are found in many natural and manufactured products, including perfumes and food products. The most common example of a natural aldehyde is formaldehyde, which is produced by plants and animals.
To learn more about Aldehyde
https://brainly.com/question/17101347
#SPJ4
what ionic compound is gold found in
Answers
Gold is found in various ionic compounds, but one of the most well-known and commercially significant compounds is gold chloride, also known as auric chloride or gold(III) chloride.
The chemical formula for gold chloride is AuCl₃. Gold chloride is an ionic compound composed of gold cations (Au³⁺) and chloride anions (Cl-). It is a yellow-orange solid that is highly soluble in water. Gold chloride can be formed by reacting the gold metal with chlorine gas or by dissolving the gold metal in aqua regia, which is a mixture of concentrated nitric acid and hydrochloric acid.
Gold chloride has several uses and applications. It is commonly used in the field of nanotechnology for the synthesis of gold nanoparticles. These nanoparticles have unique optical, electronic, and catalytic properties, making them valuable in various fields such as medicine, electronics, and materials science.
In addition to gold chloride, gold can also form other ionic compounds with different anions, such as gold bromide (AuBr), gold iodide (AuI), gold sulfide (Au2S), and gold cyanide (AuCN). These compounds have their own unique properties and applications.
know more about anions here:
https://brainly.com/question/31485566
#SPJ8
Write the isotope notation for an element with 53 protons and 20 neutron?
Answers
The isotope notation : ⁷³₅₃X
Further explanationGiven
53 protons and 20 neutron
Required
The isotope notation
Solution
Isotopes are elements that have the same atomic number with a different mass number
The following element notation,
\(\large {{{A} \atop {Z}} \right X}\)
X = symbol of element
A = mass number
= number of protons + number of neutrons
Z = atomic number
= number of protons = number of electrons, on neutral elements
So the mass number of element = 53 + 20 = 73
Atomic number = 53
The symbol :
\(\large {{{73} \atop {53}} \right X}\)
How Would The IR Spectrum Of Acetylferrocene Differ From That Of Ferrocene?
Answers
The IR spectrum of Acetylferrocene will differ from that of Ferrocene because of the carbonyl stretching and bending modes that appear in the Acetylferrocene.
The IR spectrum of Acetylferrocene will differ from that of Ferrocene due to the carbonyl stretching and bending modes that appear in the Acetylferrocene.
Ferrocene and Acetylferrocene have similar IR spectra since they both have the Fe-Cp stretching and bending modes. The acetyl group of Acetylferrocene is reflected by an intense band in the 1700-1750 cm-1 range, which is due to carbonyl stretching.
In Acetylferrocene, the IR spectra are dominated by the presence of the acetyl group's vibration, resulting in a change in the frequency of stretching vibration from 200 to 220 cm−1. Another change in the IR spectra of Acetylferrocene is the presence of two bands due to C-O stretching at 1230-1260 cm-1 in addition to the appearance of a strong band due to C-H bending vibrations in the 1410-1450 cm-1 region.
Ferrocene does not have a carbonyl group, which is why it will not display the carbonyl stretching and bending vibrations in the IR spectra. This is the most significant difference between the two IR spectra. So, we can conclude that the IR spectrum of Acetylferrocene will differ from that of Ferrocene due to the presence of the carbonyl group.
Therefore, The IR spectrum of Acetylferrocene will differ from that of Ferrocene because of the carbonyl stretching and bending modes that appear in the Acetylferrocene.
Learn more about Acetylferrocene
brainly.com/question/31787348
#SPJ11
What does net force depend on
Answers
Answer:
net force depends upon the object and also the mass of that object
Answer:The acceleration of an object depends directly upon the net force acting upon the object, and inversely upon the mass of the object.
Explanation:
Nitrogen has a lower ionization energy value than ___ when moving from beryllium to neon.
1. Carbon
2. Beryllium
3. Boron
4. Fluorine
Answers
Hope this helps!
Which type of electromagnetic wave has the greatest frequency?(1 point)
a. visible light
b. radio waves
c. x-rays
d. ultra violet light
Answers
c. x-rays
My answer is that x-rays or gamma rays have the greatest (or highest) frequency waves.
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
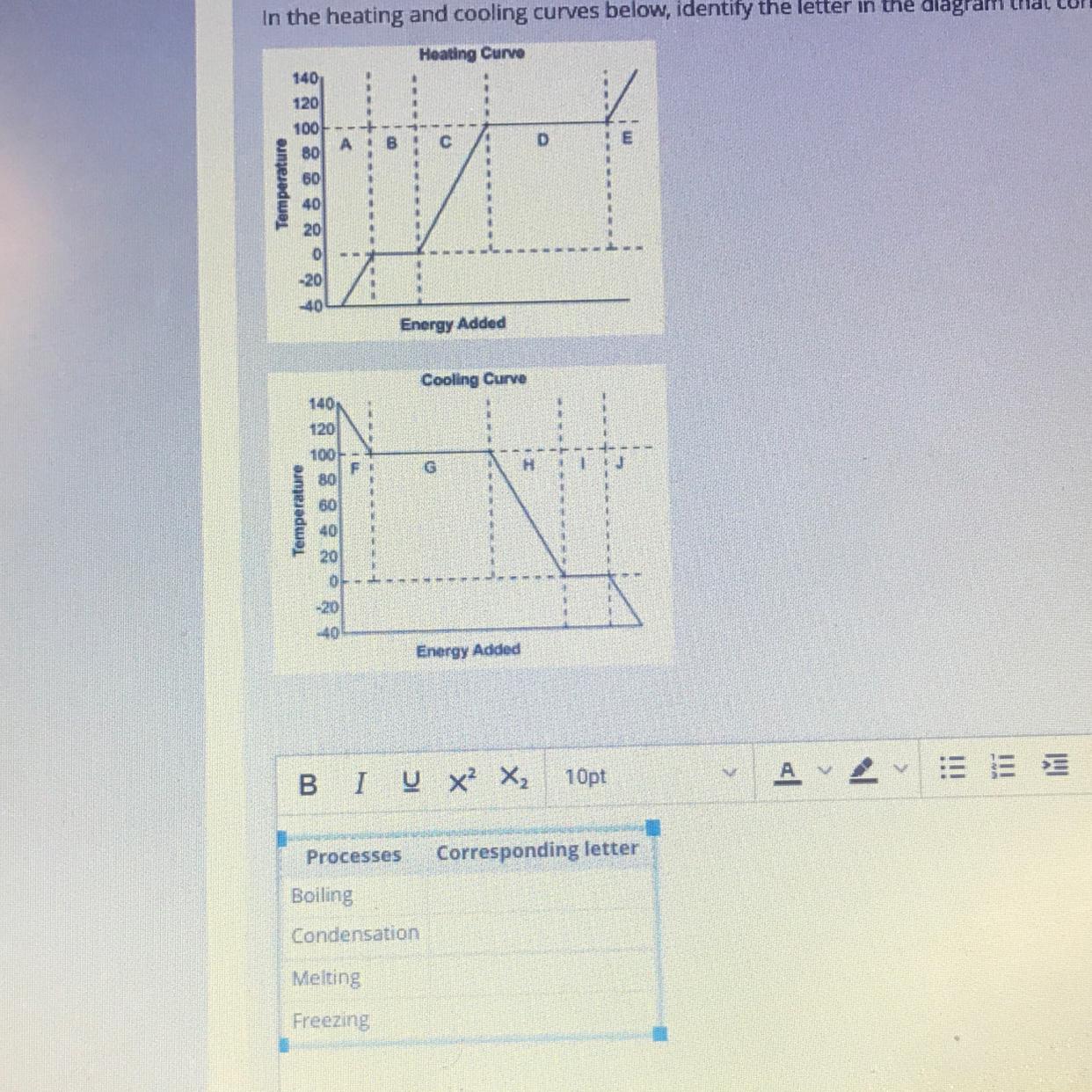
Answers
Answer:
Test for the first one is the best for
A significant amount of methane (CH4) is trapped underground or under marine sediments as Select one: a. methane hydrates. b. liquid methane. c. inorganic carbon. d. natural gas.
Answers
Methane is found under the ground or under marine sediments as natural gas which is mined along with crude oil.
Methane is an alkane and has the formula CH4. It is most commonly used as fuel. Methane is the gas that has been renowned as being responsible for explosion in coal mines.
Methane is found under the ground as natural gas which is mined along with crude oil. Large deposit of natural gas is found in many countries in Asia, Africa and the middle east.
Learn more: https://brainly.com/question/6505878
What are anthropogenic greenhouse gas emissions?
Answers
Anthropogenic greenhouse gas emissions refer to the release of greenhouse gases into the atmosphere due to human activities. These emissions mainly result from the burning of fossil fuels, deforestation, industrial processes, and agriculture. The primary greenhouse gases include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases.
1) CO2 is the most significant anthropogenic greenhouse gas, primarily emitted from the combustion of fossil fuels such as coal, oil, and natural gas. This occurs during activities like electricity production, transportation, and manufacturing processes. Deforestation also contributes to CO2 emissions, as trees and plants absorb CO2 while they grow, and release it back into the atmosphere when they are cut down or burned.
2) Methane is another important greenhouse gas, mainly produced during the decomposition of organic matter in landfills, the digestive processes of livestock, and rice cultivation. The extraction and transport of fossil fuels, particularly natural gas, also result in methane emissions.
3) Nitrous oxide emissions primarily come from agricultural activities, such as the application of synthetic fertilizers and the management of livestock waste. Industrial processes and fossil fuel combustion also contribute to N2O emissions.
4) Fluorinated gases are synthetic chemicals used in various industrial applications, including refrigeration, air conditioning, and electronics manufacturing. Although emitted in smaller quantities, they are potent greenhouse gases with long atmospheric lifetimes.
5) Efforts to reduce anthropogenic greenhouse gas emissions include transitioning to renewable energy sources, increasing energy efficiency, and promoting sustainable land management and agriculture practices.
for more such question on Anthropogenic
https://brainly.com/question/13047496
#SPJ11
Which of the following elements has 2 electrons in the 4s sublevel?

Answers
Answer:
B. Ca
Explanation:
Let's look at the electron configurations of all four elements (I am going to write it in noble gas configuration to make it simpler):
Mg electron configuration: [Ne]3s2
Ca electron configuration: [Ar]4s2
Ar electron configuration: [Ar]
K electron configuration: [Ar] 4s1
We notice that Ca has two electrons in the 4s sublevel, which satisfies what the question is asking for.
The answer is thus B. Ca.
You want to control the temperature of an enzyme-controlled reaction that is taking place inside a flask. What is the most effective way to keep the reaction at a controlled and steady temperature?
Answers
Use of thermostat is the most effective way to keep the reaction at a control temperature.
What is the most effective way to keep the reaction in control?The reaction takes place in a thermostat is the most effective way to keep the reaction at a controlled and steady temperature because thermostat regulates the temperature of that solution which is placed in it. If the temperature goes higher then the extra heat is released from the system.
So we can conclude that use of thermostat is the most effective way to keep the reaction at a control temperature.
Learn more about temperature here: https://brainly.com/question/24746268
#SPJ1
Calculate the percent ionization of an aqueous solution containing 0.13 m formic acid, hco2h(aq), and 0.11 m potassium formate, hco2k(aq). for formic acid, ka = 1.8
Answers
The percent ionization of formic acid increases by 38.46% when potassium formate is added to the solution.
Here is the calculation of the percent ionization of an aqueous solution containing 0.13 M formic acid, HCOOH(aq), and 0.11 M potassium formate, HCOOK(aq). For formic acid, Ka = 1.8:
Initial concentrations:
HCOOH = 0.13 M
HCOOK = 0.11 M
Equilibrium concentrations:
HCOOH = 0.13 - x M
HCOOK = 0.11 + x M
H+ = x M
HCOO- = x M
Where x is the amount of formic acid that ionizes.
Ka = [H+][HCOO-] / [HCOOH]
1.8 = x^2 / 0.13 - x
x^2 + 2.34x - 2.34 = 0
(x + 4.68)(x - 0.5) = 0
x = 0.5
Therefore, the percent ionization of the formic acid solution is:
percent ionization = (x / [HCOOH]) * 100% = (0.5 / 0.13) * 100% = 38.46%
The presence of potassium formate in the solution increases the percent ionization of formic acid because potassium formate is a base. Bases react with acids to form water and a salt. In this case, potassium formate reacts with formic acid to form potassium hydrogen formate and water. This reaction removes some of the formic acid from the solution, which increases the concentration of the hydrogen ions and the percent ionization of the formic acid solution.
To learn more about ionization: https://brainly.com/question/1602374
#SPJ11
HELP ME PLEASE WILL GIVE MORE POINTS AND BRAINLIEST!!
The specific heat of aluminum is 0.900 J/gºC. How much energy is needed to raise the temperature of a 20.0 g block of aluminum from 22.8°C to 94.6°C?
Answers
✅Show work regardless if student got answer correct or incorrect
q = mC∆T
q = (30.0g)(0.900J/goC)(50oC)
q = 1350 J
So, the right answer is 1350 J
IamSugarBee◽