Write balanced net ionic equations for the reaction that takes place between H2O2(aq) and MnO4-(aq), in acid medium:
a) the oxidation half-reaction.
b) the reduction half-reaction.
c) the overall reaction.
Answers
Here are the balanced net ionic equations for the reaction between H2O2(aq) and MnO4-(aq) in an acid medium
a) Oxidation half-reaction goes as:
H2O2(aq) → O2(g) + 2H+(aq) + 2e-
b) Reduction half-reaction goes as:
MnO4-(aq) + 8H+(aq) + 5e- → Mn2+(aq) + 4H2O(l)
c) The overall reaction is:
2MnO4-(aq) + 5H2O2(aq) + 6H+(aq) → 2Mn2+(aq) + 5O2(g) + 8H2O(l)
Note that in order to balance the net ionic equations, the number of electrons lost in the oxidation half-reaction must equal the number of electrons gained in the reduction half-reaction.
Also, the overall reaction should be balanced in terms of both mass and charge.
To know more about the balanced net ionic equations, click below.
https://brainly.com/question/31814906
#SPJ11
Related Questions
A solid substance turns directly into a gas. Which term describes this change?
deposition
evaporation
melting
sublimation
Answers
Answer:
the correct answer would be D. Sublimation
Explanation:
Sublmation- solid transforming into a gas, skipping the liquid stage.
eveporation- a liquid transformimg into a gas
melting- a solid transforming into a liquid
deposition- the opposite of sublimation
hope this helps
Answer:
D) Sublimination
Explanation:
0.3043 g of pure KHP was weighed out and titrated to an end point with 15.12 mL of a NaOH solution that was approximately 0.1 M. What is the exact concentration of the NaOH titrant
Answers
The exact concentration of the NaOH titrant is approximately 20.22 M.The exact concentration of the NaOH titrant can be calculated using the equation:
M1V1 = M2V2
Where M1 is the concentration of the NaOH solution, V1 is the volume of the NaOH solution used, M2 is the concentration of the KHP (potassium hydrogen phthalate), and V2 is the mass of the KHP.
Given that the mass of KHP is 0.3043 g and the volume of NaOH used is 15.12 mL, we can convert the volume to liters by dividing by 1000 (since 1 mL = 0.001 L).
V1 = 15.12 mL ÷ 1000
= 0.01512 L
Now we can rearrange the equation and solve for M1:
M1 = (M2 × V2) ÷ V1
Since KHP is a monoprotic acid, the molar mass of KHP is 204.23 g/mol, we can calculate the number of moles of KHP:
n(KHP) = mass(KHP) ÷ molar mass(KHP)
n(KHP) = 0.3043 g ÷ 204.23 g/mol
= 0.001493 mol
Now we can calculate the concentration of the NaOH titrant:
M1 = (0.001493 mol × 204.23 g/mol) ÷ 0.01512 L
M1 ≈ 20.22 M
Therefore, the exact concentration of the NaOH titrant is approximately 20.22 M.
To know more about titrant visit:
https://brainly.com/question/29341590
#SPJ11
Which of the following statements about the neuromuscular junction acetylcholine (Ach) receptor is false?
a. The probability an ACh channel will open depends on ACh concentration.
b. The synaptic current is the sum of the net ionic currents through all activated ACh receptors.
c. The opening of the ACh receptor is all-or-none.
d. The probability that an ACh channel will open depends on membrane voltage.
e. The current through a single receptor can be measured with a patch clamp technique.
Answers
The false statement about the neuromuscular junction acetylcholine (ACh) receptor is c. The opening of the ACh receptor is all-or-none.
The degree to which the ACh receptor opens is dependent on both the amount of neuromuscular junction acetylcholine (ACh) present and the level of receptor activation; it is not an all-or-nothing response. The channel opens when ACh binds to the receptor, causing a conformational shift that permits ion flow.
The following claims are accurate:
ACh concentration affects whether an ACh channel will open, with a larger ACh concentration increasing the likelihood that the channel will open and ion flow.
The net ionic currents through all activated ACh receptors add up to make up the synaptic current, which is defined as the sum of all such currents.
Membrane voltage impacts the likelihood that an ACh channel will open: Membrane voltage has an impact on the likelihood that an ACh channel will open. In general, hyperpolarization lowers and depolarization enhances the likelihood of a channel opening.
The patch clamp method allows for the measurement of the current flowing through a single receptor, including ACh receptors. This method sheds light on the characteristics and functions of individual ion channels, including ACh receptors.
As a result, c is the erroneous assertion. All or none of the ACh receptor's opening occurs.
To know more about neuromuscular junction:
https://brainly.com/question/27526751
#SPJ4
What is the molar concentration of a solution prepared by dissolving 3.05 grams of sodium acetate in enough water to prepare 3.43 x 102 mL of the solution
Answers
Answer:
0.296 M
Explanation:
What is the molar concentration of a solution prepared by dissolving 3.05 grams of sodium acetate in enough water to prepare 3.43 x 102 mL of the solution
sodium acetate NaCH3COO has molar mas of
23 + 12 +24 + 1 +32 = 92 g/mol
3.05 g is 3.05 g/ 92 g/mol = 0.0328 moles
it is distributed over 102 mL = 0.102 L
its concentration is 0.0328/ 0.102 =0.296 M Mhttps://brainly.com/question/25586315?answering=true&answeringSource=feedPublic%2FhomePage%2F3#
what is the experimental group in the alka-seltzer experiment
Answers
The Alka-Seltzer experiment's experimental group is the group of volunteers who are given Alka-Seltzer pills to ingest.
The experiment's goal is to test the hypothesis that Alka-Seltzer pills will shorten the time it takes for stomach discomfort to go away. The control group consists of volunteers who were given a placebo, or a false drug, instead of the Alka-Seltzer pills.
The experimental group is critical to the experiment because it allows for a comparison of the outcomes seen in subjects given Alka-Seltzer pills vs those given a placebo. The hypothesis could not be tested and the results could not be appropriately understood in the absence of an experimental group.
Learn more about the Alka-seltzer experiment at
https://brainly.com/question/29106242
#SPJ4
Which organelle is like armor for the cell?
Answers
Answer:
Cell wall
Explanation:
i just searched it up
A solution of 314 grams of NaI3 in 1.18 kilograms of water. Find molality.
Answers
The solution has a molality of 0.658 mol/kg.
What is molality ?The amount of moles of solute per kilogram of solvent is known as molality (m).
We must first determine the number of moles of NaI3 in the solution in order to determine the molality of a solution containing 314 grams of NaI3 in 1.18 kilograms of water.
The formula below can be used to determine NaI3's molar mass:
Na: 1 x 22.99 = 22.99 g/mol
I: 3 x 126.90 = 380.70 g/mol
Total molar mass: 22.99 + 380.70 = 403.69 g/mol
The number of moles of NaI3 in the solution is therefore:
moles = mass/molar mass
moles = 314 g/403.69 g/mol
moles = 0.7786 mol
Next, we need to calculate the mass of water in the solution:
mass of water = 1.18 kg = 1180 g
Finally, we can determine the solution's molality:
molality = moles of solute/mass of solvent (in kg)
molality = 0.7786 mol/1.18 kg
molality = 0.658 mol/kg
Therefore, The solution has a molality of 0.658 mol/kg.
Learn more about molality here : brainly.com/question/24065939
#SPJ1
1.
What is the molarity of a 3.6 L solution with 104 g of sucrose?
Answers
Answer:
.084 M
Explanation:
Molar mass of sucrose = 342.3
The formula to find molarity is moles of solute / liters of solution
Since we need to turn grams to moles for this equation we do 104 / 342.3 = 0.304
Now we can use the equation listed above. .304 / 3.6 = 0.084 M
Is it possible for any of the earth materials scenes to be affected by both mechanical and chemical weathering? If so, describe the scene and situations.
Answers
The freezing and thawing of water within cracks and crevices can lead to mechanical weathering through frost wedging, while rainwater that percolates into the rock can react with minerals in the rock, causing chemical weathering through processes such as hydrolysis and oxidation.
Yes, it is common for earth materials to be affected by both mechanical and chemical weathering simultaneously. A prime example of such a scene is a mountainous region where exposed bedrock is subjected to both mechanical and chemical weathering. Coastal regions also provide an example of mechanical and chemical weathering acting in unison. Rocks exposed to the constant pounding of waves and sea spray are subjected to mechanical force causing physical weathering by breaking down the rock into smaller pieces, while saltwater can react with minerals in the rock and cause chemical weathering, particularly through processes like dissolution and hydration. Both of these situations work together to break down the rock, alter its appearance and composition over time.
for more such questions on weathering
https://brainly.com/question/29488611
#SPJ11
Describe how nucleic acid basis pair up
Answers
The rules of base pairing (or nucleotide pairing) are: A with T: the purine adenine (A) always pairs with the pyrimidine thymine (T) C with G: the pyrimidine cytosine (C) always pairs with the purine guanine (G)
The nucleotides in a base pair are complementary which means their shape allows them to bond together with hydrogen bonds. The A-T pair forms two hydrogen bonds. The C-G pair forms three. The hydrogen bonding between complementary bases holds the two strands of DNA together.
During the reaction of CV with NaOH, do you expect the colorimeter absorbance to change? How do you expect it to change if such a change is anticipated (i.e, increase, decrease, stay the same) as the reaction proceeds? Explain
Answers
It is anticipated that as the reaction proceeds the concentration of CV decreases and the absorbance of solution is also expected to decrease.
The reaction between CV (Cyanide) and NaOH (Sodium Hydroxide) will result in the production of NaCN (Sodium Cyanide) and H2O. The colorimeter absorbance of the solution is expected to change as the reaction proceeds. The change in absorbance would depend on the concentration of CV in the solution, and the reaction rate between CV and NaOH.
In general, as the reaction progresses and the concentration of CV decreases, the absorbance of the solution is expected to decrease. The decrease in absorbance would occur because the concentration of the absorbing species (CV) is decreasing, leading to a decrease in the amount of light absorbed by the solution.
It is important to note that the exact change in absorbance would depend on the specific conditions of the reaction, including the initial concentration of CV, the reaction rate, and the wavelength of light used by the colorimeter.
Learn more about colorimeter here:
https://brainly.com/question/29385087
#SPJ4
Identify the following disaccharides by dragging the names to the boxes under the structures. O lactose O celllobiiose O maltose O sukrose
Answers
These common disaccharides, lactose, cellobiose, maltose, and sucrose have distinct structures and biological functions, and play important roles in energy storage and cellular processes.
Biological functionsIn biochemistry, disaccharides are molecules composed of two monosaccharide units joined by a glycosidic bond. Four common disaccharides are lactose, cellobiose, maltose, and sucrose.
Lactose is composed of a galactose unit and a glucose unit, and is commonly found in milk. Cellobiose is made up of two glucose units and is a component of cellulose. Maltose is composed of two glucose units and is formed during starch digestion.
Sucrose is made up of a glucose unit and a fructose unit, and is commonly found in table sugar. These disaccharides have different biological functions and play an important role in energy storage and cellular processes.
Threrefore,
lactose -> Galactose - Glucosecellobiose -> Glucose - Glucosemaltose -> Glucose - Glucosesucrose -> Glucose - FructoseLearn more about biological functions: ://brainly.com/question/29396589
#SPJ11
What occurs as the atomic number of the elements in Period 2 increases?
1)The nuclear charge of each successive atom decreases, and the atomic radius decreases.
2)The nuclear charge of each successive atom decreases, and the atomic radius increases.
3)The nuclear charge of each successive atom increases, and the atomic radius decreases
4)The nuclear charge of each successive atom increases, and the atomic radius increases.
Answers
Answer:
3)The nuclear charge of each successive atom increases, and the atomic radius decreases
Explanation:
As we move from left to right across the periodic table the number of valance electrons in an atom increase. The atomic size tend to decrease in same period of periodic table because the electrons are added with in the same shell. When the electron are added, at the same time protons are also added in the nucleus. The positive charge is going to increase and this charge is greater in effect than the charge of electrons. This effect lead to the greater nuclear attraction. The electrons are pull towards the nucleus and valance shell get closer to the nucleus. As a result of this greater nuclear attraction atomic radius decreases and ionization energy increases because it is very difficult to remove the electron from atom and more energy is required.
the process by which the atoms of one or more substances are rearranged to form different substances is called a
Answers
Answer:
Chemical reaction
Explanation:
This process is called a chemical reaction. A chemical reaction is where atoms are rearranged to create various compounds, so the atoms can either be formed or broken.
For example, in this equation for the formation of water:
2H2(g) + O2(g) → 2H2O(l)
Here hydrogen and oxygen both combine to form water. This new compound, water, that has formed is what makes it a chemical reaction.
In this equation for the decomposition of calcium carbonate:
CaCO3(s) → CaO(s)+ CO2(g)
Here, CaCO3 has been broken down into CaO and CO2, which takes place as a chemical reaction.
For hydrogen atoms, the wave function for the state n = 3,l = 0, m_l = 0 is given below psi_300 = 1/81 squareroot 3 pi (1/a_0)^3/2 (27 - 18 sigma + 2 sigma^2)e^-sigma^2)e^-sigma/3 In this equation sigma = r/a_0 and a_0 is the Bohr radius (5.29 times 10^-11 m). Calculate the position of the nodes for this wave function. higher value & lower value
Answers
To calculate the position of the nodes for the wave function of a hydrogen atom with quantum numbers n = 3, l = 0, and m_l = 0, given by psi_300 = 1/81√(3π)(1/a_0)^3/2 (27 - 18σ + 2σ^2)e^(-σ/3), where σ = r/a_0 and a_0 is the Bohr radius (5.29 x 10^-11 m).
1. To find the nodes, we need to solve the equation psi_300 = 0.
2. Focus on the radial part of the wave function, which is (27 - 18σ + 2σ^2).
3. Set the radial part equal to 0: 27 - 18σ + 2σ^2 = 0.
4. Solve this quadratic equation for σ, either by factoring or using the quadratic formula.
5. The quadratic formula is σ = (-b ± √(b^2 - 4ac))/(2a), where a = 2, b = -18, and c = 27.
6. Calculate σ values: σ1 = 3 and σ2 = 9.
7. Convert these values of σ back to positions (r) using the relation σ = r/a_0.
8. Calculate r values: r1 = σ1 * a_0 and r2 = σ2 * a_0.
Summary:
For the hydrogen atom with given wave function psi_300, the positions of the nodes are r1 = 3 * a_0 ≈ 1.59 x 10^-10 m (lower value) and r2 = 9 * a_0 ≈ 4.76 x 10^-10 m (higher value).
learn more about wave function click here:
https://brainly.com/question/28447252
#SPJ11
Fill in the table with the mass of each sample. Use the periodic table to find the molar mass of each compound. Then calculate moles and particles. Periodic TableThe two compounds have the same number of moles. Compare how the number of moles relates to the masses and number of particles for each compound.

Answers
1) First, let's calculate the molar mass of NaCl and CoCl₂:
Atomic mass of:
Na - 22.99 g/mol
Cl - 35.45 g/mol
Co - 58.93 g/mol
So for NaCl:
22.99 + 35.45 = 58.44 g/mol
Molar mass of NaCl = 58.44 g/mol
For CoCl₂:
58.93 + (2x35.45) = 129.83 g/mol
Molar mass of CoCl₂: 129.83 g/mol
2) Now let's find out the number of moles of each sample. For this, we use the following equation:
moles = mass / molar mass
NaCl:
mole = 116.8 / 58.44
mole = 1.999 moles of NaCl
CoCl₂:
mole = 259.7 / 129.83
mole = 2.000 moles of CoCl₂
3) To find the amount of particles, we use the Avogadro's constant. Avogadro's constant is a constant of proportion between the amount of matter and the number of entities that are linked to that amount. These entities can be atoms, molecules, ions, electrons, protons, neutrons.
In this case, we have the same number of moles, so we will have the same number of particles for both NaCl and CoCl₂.
Determine the volume, in liters, of 3.2 mol of CO2 gas at STP.
Answers
Answer:
71.7 L
Explanation:
Using the ideal gas equation;
PV = nRT
Where;
P = pressure (atm)
V = volume (L)
n = number of moles (mol)
R = gas law constant (0.0821 Latm/Kmol)
T = temperature (K)
According to the information provided in this question;
P = 1 atm (STP)
V = ?
n = 3.2mol
T = 273K (STP)
Using PV = nRT
V = nRT/P
V = 3.2 × 0.0821 × 273/1
V = 71.7 L
What mass of H2 forms when
35.25 g of Al reacts with excess
hydrochloric acid?
2AI+ 6HCI→ 2AlCl3 + 3H₂
Al: 26.98 g/mol
H₂: 2.02 g/mol
[?] g H₂

Answers
35.25/26.98 (mass reacted/molar mass of Al)
2) Find number of moles of H2 produced:
Number of mol. of Al x 3/2 (based on stoichiometry)
3) Find mass of H2 formed:
Number of mol. of H2 x molar mass of H2 = 3.9588mol = 4 mol (estimated value)
a 17.27 gram sample of aluminum initially at 92 oc is added to a container containing water. the final temperature of the metal is 25.1 oc. what is the total amount of energy in joules added to the water? what was the energy lost by the metal?
Answers
The heat released by metal and added to water is the same which is 1039.83 joule.
The equilibrium condition of the system depends on the heat released from both gold and water. The total heat received by the system will equal to total heat released by objects. It should follow
Q released = Q received
The heat can be defined by
Q = m . c . ΔT
where Q is heat, m is mass, c is the specific heat constant and ΔT is the change in temperature.
The given parameters are
m = 17.27 g = 0.01727 kf
T1 = 92 ⁰C
T2 = 25.1 ⁰C
c = 900 J/kg⁰C
By substituting the given parameters, we can calculate the heat
Q = m . c . ΔT
Q = 0.01727 . 900 . (92 - 25.1)
Q = 1039.83 joule
Find more on heat at: https://brainly.com/question/8925446
#SPJ4
Mot radioactive element have
1. More neutron than proton
2. More proton than neutron
3. More electron than proton
4. Equal number of proton and neutron
Answers
According to the given statement Most radioactive element have more proton than neutron.
Which is radioactive element?For instance, the Earth's crust naturally contains the radioactive elements uranium and thorium. These two elements progressively alter their forms over millions of years, leading to the production of decay byproducts like radon and radium. Energy is released during this process. Alpha radiation is one type of this energy.
How do you recognize radioactive substances?By monitoring the activity of the relevant radioisotopes, it is possible to identify naturally occurring radioactive materials. When it comes to radioactive materials with brief half-lives, these measurements are very useful. Despite being the most ionizing, alpha particles have a relatively shallow penetration depth; they cannot reach the skin's higher layers.
To know more about Radioactive element visit:
https://brainly.com/question/17551878
#SPJ4
how much water will you need to add to your working solution to get to a 100 ml total volume of a 0.03m nacl solution?
Answers
Answer:
The solution is made by adding 4.38 g NaCl to a 250-mL volumetric flask. About 100 mL of water are added and when all the NaCl dissolves water is added up ...
15. What are the benefits to having a nuclear power plant nearby?
Answers
Low-cost energy. Although building nuclear power plants has a high initial cost, it's relatively cheap to produce energy from them and they have low operating costs.
Reliable.
Zero carbon emissions.
Promising future energy supply.
High energy density.
What are some foods that require dry ice
Answers
A 10.0 ml portion of 0.010 m hcl is diluted by adding it to100.0 ml of water. what is the ph of the solution
Answers
From the calculation, the pH of the solution after dilution is 3.
What is the pH?The pH is the hydrogen ion concentration of the solution. Now we know that;
C1 = 0.010 m
V1 = 10.0 ml
V2 = 10.0 ml + 100.0 ml = 110 ml
C2 = ?
C1V1 = C2V2
C2 = C1V1 /V2
C2 = 0.010 m * 10.0 ml / 110 ml
C2 = 0.00091 M
pH = -log[0.00091 M]
pH = 3
Learn more about pH:https://brainly.com/question/15289741
#SPJ1
Why do you have to have a certain number of reactants on one side?
Answers
Answer:Because you need that certain number and double the number of bread to get the reactants off of the sides
Explanation: 4 bread reactants- 2meat reactants- 2 cheese reactants= 2 products
The reason for the presence of a certain number of reactants on one side is to ensure that the chemical equation is balanced.
In a balanced chemical equation, the number of atoms of each element on the reactant side must be equal to the number of atoms of that element on the product side.
Consider the equation:
\(2H_2 + O_2 - > 2H_2O.\)
This equation represents the reaction of hydrogen gas (\(H_2\)) with oxygen gas (\(O_2\)) to form water (\(H_2O\)).
To balance this equation, double the coefficient in front of \(H_2O\) on the product side, resulting in 4 hydrogen atoms and 2 oxygen atoms on both sides.
Therefore, having a certain number of reactants on one side is necessary to ensure that a chemical equation is balanced, satisfying the law of conservation of mass.
To know more about the balanced chemical equation, visit:
https://brainly.com/question/29130807
#SPJ3
which statmemt is true for most chemical reactions an energy change occurs during the breaking and forming of bonds
Answers
An energy change occurs during the breaking and forming of bonds is true for most chemical reactions an energy change occurs during the breaking and forming of bonds .
Chemical bonds between atoms and molecules can break and form during chemical reactions. Existing bonds must be broken in order for new ones to form, which releases energy during the process. The enthalpy change or overall energy change during a chemical reaction is typically expressed as the energy difference between the products and the reactants.
Depending on whether energy is absorbed or released during a reaction, the enthalpy change can either be positive or negative. The reaction is exothermic and energy is released if the enthalpy of the products is lower than the enthalpy of the reactants. The reaction is endothermic and energy is absorbed if the enthalpy of the products is greater than the enthalpy of the reactants.
The question is incomplete, complete question will be "Which statement is true for most chemical reactions?
An energy change occurs during the breaking and forming of bonds.
The internal energy of the system increases during a reaction.
Energy is released during the formation of reactants.
The enthalpy of the products is higher than the enthalpy of the reactants."
Learn more about chemical reactions at:
brainly.com/question/29762834
#SPJ1
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
when you hold a metal coat hanger in a camp fire to roast a marshmallow, the coat hanger might get too hot to hold. what type of heat transfer is occurring between the metal coat hanger and your hand? explain what is happening at the molecular level that causes your hand to feel hot.
Answers
Answer:
Heat transfer by conduction
If 500.0 mL of 0.450 M sodium phosphate is reacted with an excess of iron (II) nitrate solution, how many grams of iron (II) phosphate are produced?
Answers
Answer:
If 500.0 mL of 0.450 M sodium phosphate is reacted with an excess of iron (II) nitrate solution, how many grams of iron (II) phosphate are produced?
idk
Explanation:
Answer:
164.2726 g
The ratio in the equation is 3:1 so the limit reaction ratio is 3:1 therefore, 0.392456 mol of were reaction.
Brainlist pls!
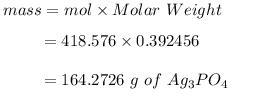
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work